All Photos(2)
About This Item
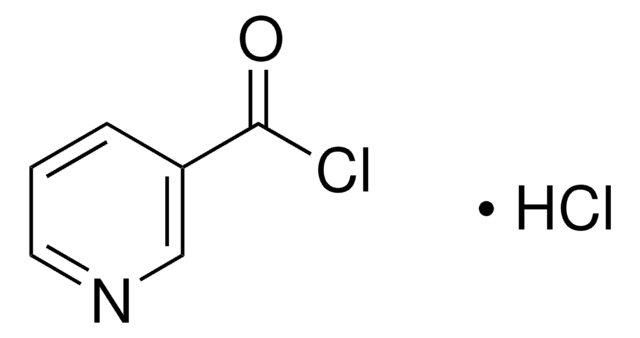
Empirical Formula (Hill Notation):
C6H3Cl2NO
CAS Number:
Molecular Weight:
176.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
48-51 °C (lit.)
functional group
acyl chloride
chloro
SMILES string
ClC(=O)c1ccc(Cl)nc1
InChI
1S/C6H3Cl2NO/c7-5-2-1-4(3-9-5)6(8)10/h1-3H
InChI key
FMEBIWNKYZUWFV-UHFFFAOYSA-N
General description
6-Chloronicotinoyl chloride undergoes esterification reaction with diethylene glycol and pentaethylene glycol.
Application
6-Chloronicotinoyl chloride may be used to synthesize:
- [3H]imidacloprid (a candidate radioligand)
- [3H]acetamiprid
- N,N′-(1,4-phenylene)bis(6-(4-aminophenoxy) nicotinamide)
- 3-acetyl-6-chloropyridine
- 1,3-dipropyl-8(6-chloro-3-pyridyl)xanthine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinzo Kagabu et al.
Bioscience, biotechnology, and biochemistry, 67(5), 980-988 (2003-07-02)
The asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine, was prepared, and the absolute configurations of the enantiomers were determined by an X-ray analysis. The insecticidal activity against the housefly measured with metabolic inhibitors showed the (S) enantiomer to be slightly more active than
Nicotinic acid crown ethers. Synthesis and structural characterization of polyethereal macrocyclic lactones from 6-chloronicotinic acid.
Newkome GR, et al.
The Journal of Organic Chemistry, 45(26), 5423-5425 (1980)
P J Scammells et al.
Journal of medicinal chemistry, 37(17), 2704-2712 (1994-08-19)
This report describes the synthesis of 29 xanthines containing a chemoreactive chloroaryl, beta-chloroethylamino, alpha,beta-unsaturated carbonyl, bromoacetyl, 3-(fluorosulfonyl)benzoyl, or 4-(fluorosulfonyl)benzoyl group as part of an exocyclic 1-, 3-, or 8-substituent. The xanthines inhibited the binding of [3H]-8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX) to the A1
Preparation of thermally stable, low dielectric constant, pyridine-based polyimide and related nanofoams.
Aram E and Mehdipour-Ataei S.
Journal of Applied Polymer Science, 128(6), 4387-4394 (2013)
[3H] imidacloprid: Synthesis of a candidate radioligand for the nicotinic acetylcholine receptor.
Latli B and Casida JE.
Journal of Labelled Compounds & Radiopharmaceuticals, 31(8), 609-613 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service