471526
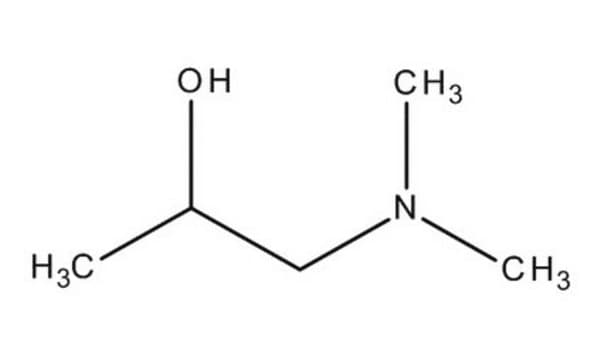
1-Dimethylamino-2-propanol
≥99%
Synonym(s):
N,N-Dimethylisopropanolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH2N(CH3)2
CAS Number:
Molecular Weight:
103.16
Beilstein:
1209244
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.6 (vs air)
Quality Level
vapor pressure
8 mmHg ( 20 °C)
Assay
≥99%
refractive index
n20/D 1.419 (lit.)
bp
121-127 °C (lit.)
density
0.837 g/mL at 25 °C (lit.)
SMILES string
CC(O)CN(C)C
InChI
1S/C5H13NO/c1-5(7)4-6(2)3/h5,7H,4H2,1-3H3
InChI key
NCXUNZWLEYGQAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Dimethylamino-2-propanol (1DMA2P, DMAPH), a tertiary amine, is a dimethylamino-alcohol having high boiling point. It is a potent protector against mechlorethamine cytotoxicity and inhibitor of choline uptake. Kinetics of homogeneous reaction of carbon dioxide (CO2) with 1-dimethylamino-2-propanol in water has been investigated using stopped-flow technique.
Application
1-Dimethylamino-2-propanol solution may be used in the synthesis of the following:
- novel unsymmetrical 2,3,9,10,16,17,23-heptakis(alkoxyl)-24-mono(dimethylaminoalkoxyl)phthalocyanine compounds
- homoleptic nickel(II) aminoalkoxide
- Adduct with cobalt(II) 2,4-pentanedionate (acac)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
78.8 °F - DIN 51755 Part 1
Flash Point(C)
26 °C - DIN 51755 Part 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S A Naujokaitis et al.
Journal of pharmaceutical sciences, 73(1), 34-39 (1984-01-01)
The structure-activity relationships of 2-dimethylaminoethanol and its analogues as protectors against mechlorethamine cytotoxicity and as inhibitors of choline uptake were evaluated. Of a series of inhibitors and protectors, 2-dimethylaminoethanol was the most potent inhibitor of choline uptake and the most
Synthesis and molecular structures of cobalt (II) ?-diketonate complexes as new MOCVD precursors for cobalt oxide films.
Pasko S, et al.
Polyhedron, 23(5), 735-741 (2004)
Zhaopin Bai et al.
Inorganic chemistry, 49(19), 9005-9011 (2010-09-04)
A new pathway by means of transetherification has been developed to synthesize novel unsymmetrical 2,3,9,10,16,17,23-heptakis(alkoxyl)-24-mono(dimethylaminoalkoxyl)phthalocyanine compounds. Cyclic tetramerization of 4,5-di(alkoxyl)phthalonitrile in refluxing dimethylamino-alcohol with high boiling point such as dimethylaminoethanol (DMAE) and 1-dimethylamino-2-propanol in the presence of lithium and pyrazino[2,3-f][1,10]phenanthroline-2,3-dicarbonitrile
Soluble Ni II alkoxides based on dimethylaminoisopropoxide ligands: molecular structure of [Li (Pr i OH) Ni (η 2-OR)2 Cl]2 and of cis-NiCl2 (ROH)2 (R= CHMeCH2NMe2).
Hubert-Pfalzgraf LG, et al.
Polyhedron, 16(24), 4197-4203 (1997)
Kinetics of carbon dioxide (CO 2) with ethylenediamine, 3-amino-1-propanol in methanol and ethanol, and with 1-dimethylamino-2-propanol and 3-dimethylamino-1-propanol in water using stopped-flow technique
Kadiwala S, et al.
Chemical Engineering Journal, 179, 262-271 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![2-[2-(Dimethylamino)ethoxy]ethanol 98%](/deepweb/assets/sigmaaldrich/product/structures/194/219/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99/640/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99.png)

![1-[Bis[3-(dimethylamino)propyl]amino]-2-propanol 98%](/deepweb/assets/sigmaaldrich/product/structures/228/232/cb6938f1-8d46-4514-b5dc-8048ce8f3bf0/640/cb6938f1-8d46-4514-b5dc-8048ce8f3bf0.png)


