404586
Sinapyl alcohol
technical grade, 80%
Synonym(s):
4-Hydroxy-3,5-dimethoxycinnamyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
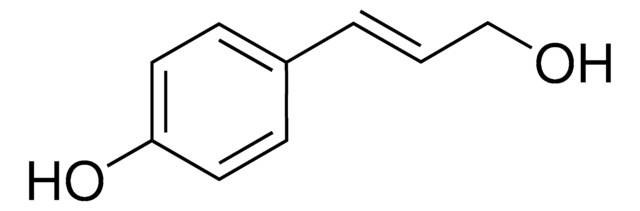
HOC6H2(OCH3)2CH=CHCH2OH
CAS Number:
Molecular Weight:
210.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
80%
form
solid
mp
61-65 °C (lit.)
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
COc1cc(\C=C\CO)cc(OC)c1O
InChI
1S/C11H14O4/c1-14-9-6-8(4-3-5-12)7-10(15-2)11(9)13/h3-4,6-7,12-13H,5H2,1-2H3/b4-3+
InChI key
LZFOPEXOUVTGJS-ONEGZZNKSA-N
General description
Sinapyl alcohol, a monolignol, is a primary lignin monomer. It has been evaluated for anti-inflammatory and antinociceptive activities. It participates in the initial stages in the biosynthesis of lignin. Coupling reactions of sinapyl alcohol and sinapyl p-hydroxybenzoate has been reported. Preparation of sinapyl alcohol by selective 1,2-reduction of corresponding cinnamate esters using diisobutylaluminium hydride as reducing agent has been studied.
Application
Sinapyl alcohol may be employed in the preparation of lignin, a highly stable biopolymer.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun Shigeto et al.
Journal of plant research, 130(1), 203-210 (2016-11-27)
Most of the known 4-coumarate:coenzyme A ligase (4CL) isoforms lack CoA-ligation activity for sinapic acid. Therefore, there is some doubt as to whether sinapic acid contributes to sinapyl alcohol biosynthesis. In this study, we characterized the enzyme activity of a
A possible mechanism for the oxidation of sinapyl alcohol by peroxidase-dependent reactions in the apoplast: enhancement of the oxidation by hydroxycinnamic acids and components of the apoplast.
Takahama U, et al.
Plant Physiology, 37(4), 499-504 (1996)
Eng-Kiat Lim et al.
FEBS letters, 579(13), 2802-2806 (2005-05-24)
This study describes the substrate recognition profile of UGT72E1, an UDP-glucose:glycosyltransferase of Arabidopsis thaliana that is the third member of a branch of glycosyltransferases, capable of conjugating lignin monomers and related metabolites. The data show that UGT72E1, in contrast to
Toshiyuki Tanaka et al.
Plant physiology, 178(2), 552-564 (2018-08-22)
Green leaf volatiles (GLVs), including six-carbon (C6) aldehydes, alcohols, and esters, are formed when plant tissues are damaged. GLVs play roles in direct plant defense at wound sites, indirect plant defense via the attraction of herbivore predators, and plant-plant communication.
Farzaneh Kordbacheh et al.
PloS one, 13(5), e0196843-e0196843 (2018-05-09)
Excessive or insufficient angiogenesis is associated with major classes of chronic disease. Although less studied, small molecules which can promote angiogenesis are being sought as potential therapeutics for cardiovascular and peripheral arterial disease and stroke. Here we describe a bioassay-directed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![4-[2-(4-Allyl-2,6-dimethoxyphenoxy)-1-hydroxypropyl]-2-methoxyphenol ≥95% (LC/MS-ELSD)](/deepweb/assets/sigmaaldrich/product/structures/103/717/7c015be4-f4aa-4720-b290-f5eaaaae5de4/640/7c015be4-f4aa-4720-b290-f5eaaaae5de4.png)


