392715
N-Fluorobenzenesulfonimide
97%
Synonym(s):
N-Fluorodi(benzenesulfonyl)amine, N-Fluorodibenzenesulfonimide, NFSI
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C6H5SO2)2NF
CAS Number:
Molecular Weight:
315.34
Beilstein:
5348902
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
110 °C (dec.) (lit.)
SMILES string
FN(S(=O)(=O)c1ccccc1)S(=O)(=O)c2ccccc2
InChI
1S/C12H10FNO4S2/c13-14(19(15,16)11-7-3-1-4-8-11)20(17,18)12-9-5-2-6-10-12/h1-10H
InChI key
RLKHFSNWQCZBDC-UHFFFAOYSA-N
Related Categories
General description
N-Fluorobenzenesulfonimide (NFSi) is a commonly used electrophilic fluorinating agent in organic synthesis to introduce fluorine into neutral organic molecules. It is also used to fluorinate nucleophilic substrates such as reactive organometallic species and malonate anions.
NFSi can be synthesized by the reaction of benzenesulfonimide with fluorine.
NFSi can be synthesized by the reaction of benzenesulfonimide with fluorine.
Application
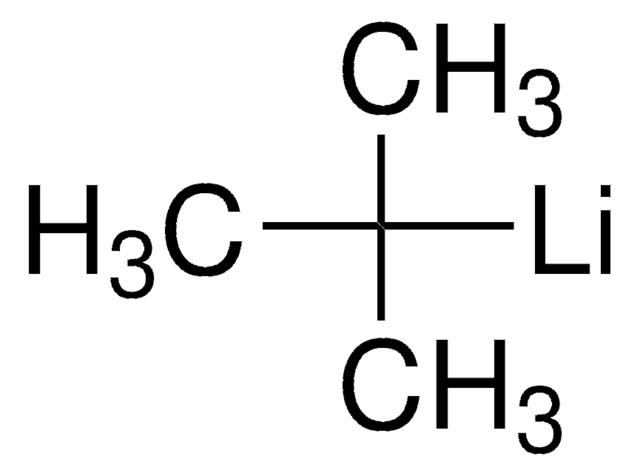
Reagent employed in a palladium-catalyzed enantioselective fluorination of t-butoxycarbonyl lactones and lactams. Also used in the electrophilic difluorination of dihalopyridines with butyl lithium and in the direct conversion of alcohols to dibenzenesulfonamides with triphenylphosphine.
Stable, easy-to-handle, crystalline material which readily transfers F+ to enolates and carbanions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Toshiaki Suzuki et al.
The Journal of organic chemistry, 72(1), 246-250 (2006-12-30)
An efficient catalytic enantioselective fluorination of tert-butoxycarbonyl lactones and lactams is reported. Reactions of the lactone substrates proceeded smoothly in an alcoholic solvent with a catalytic amount of chiral Pd(II) complex. In the case of the less acidic lactam substrates
N-fluorobenzenesulfonimide
Bizet V
Synlett, 23(18), 2719-2720 (2012)
Anthony A Fodor et al.
The Journal of general physiology, 127(6), 755-766 (2006-06-01)
The study of ion channel function is constrained by the availability of structures for only a small number of channels. A commonly used bioinformatics technique is to assert, based on sequence similarity, that a domain within a channel of interest
Tetrahedron Letters, 47, 8457-8457 (2006)
N-Fluorobenzenesulfonimide
Poss AJ
Encyclopedia of Reagents for Organic Synthesis, Second Edition, 23(18), 2719-2720 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)