391794
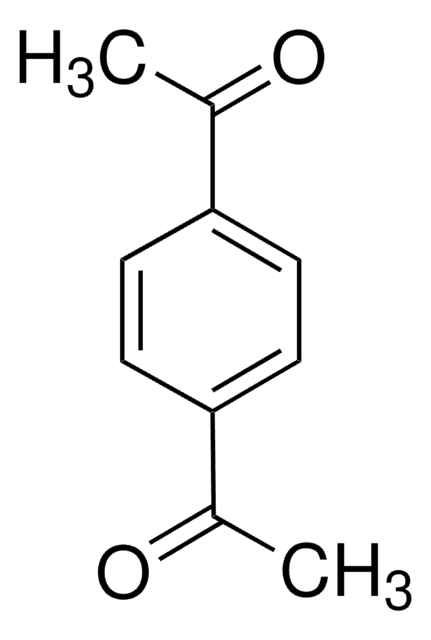
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone
99%
Synonym(s):
4,6-Diacetylresorcinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H2(COCH3)2
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
178-180 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cc(C(C)=O)c(O)cc1O
InChI
1S/C10H10O4/c1-5(11)7-3-8(6(2)12)10(14)4-9(7)13/h3-4,13-14H,1-2H3
InChI key
GEYCQLIOGQPPFM-UHFFFAOYSA-N
Related Categories
General description
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) is a bifunctional carbonyl compound. Its synthesis by acetylating resorcinol in the presence of zinc chloride has been reported. The crystal structure of DAR has been studied.
Application
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) may be used in the synthesis of the following:
- Schiff base ligands
- hexadentate chalcogenated bisimine ligands
- 1,5-benzodiazepines
- ketimine of chitosan
- mannich bases
- hydrazone ligands
- thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands
- binuclear cobalt(II) and copper(II) complexes
- europium (III) complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 428-436 (2009-12-08)
Mono- and binuclear VO(IV), Ce(III), Th(IV) and UO(2)(VI) complexes of thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands derived from 4,6-diacetylresorcinol were synthesized. The structures of these complexes were elucidated by elemental analyses, IR, UV-vis, ESR, (1)H NMR and mass spectra as well
Cahit Demetgül
Carbohydrate polymers, 89(2), 354-361 (2012-06-20)
In this study, a new chitosan derivative (ketimine) was synthesized by condensation of chitosan with 4,6-diacetylresorcinol (DAR) at heterogeneous medium. The ketimine derivative of chitosan (DAR-chitosan) was characterized by elemental (C, H, N), spectral (DR-UV-vis and FT-IR spectroscopy), structural (powder
A Facile Synthesis of 2-Benzoyl-6-Hydroxy-3-Methyl-5-(2-Substituted-2, 3-Dihydro-1H-1,5-Benzodiazepin-4-YL) Benzo [b] Furans.
Reddy K, et al.
Synthetic Communications, 30(10), 1825-1836 (2000)
Structure of 4, 6-diacetylresorcinol.
Kokila MK, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1133-1134 (1992)
Mohammed Sardaryar Khan et al.
Acta poloniae pharmaceutica, 67(3), 261-266 (2010-06-09)
In the present study, a series of Mannich bases was synthesized by condensing 4,6-diacetylresorcinol with formaldehyde and some selected secondary amines following the Mannich reaction conditions. Findings revealed that Mannich reaction did not take place at the acetyl function but
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service