All Photos(1)
About This Item
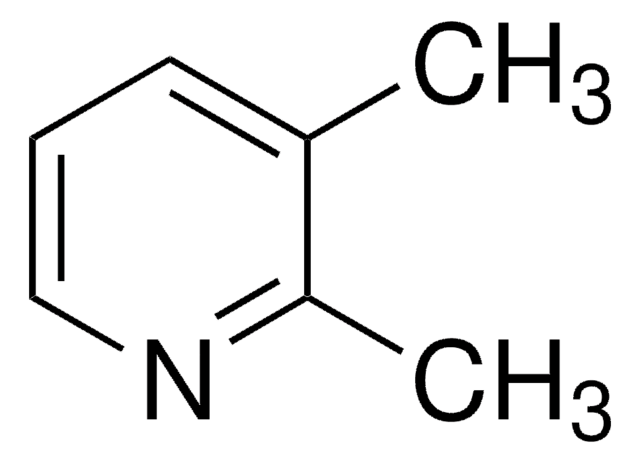
Empirical Formula (Hill Notation):
C7H9N
CAS Number:
Molecular Weight:
107.15
Beilstein:
105690
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
refractive index
n20/D 1.497 (lit.)
bp
143-145 °C (lit.)
mp
−6 °C (lit.)
density
0.92 g/mL at 25 °C (lit.)
SMILES string
Cc1cccc(C)n1
InChI
1S/C7H9N/c1-6-4-3-5-7(2)8-6/h3-5H,1-2H3
InChI key
OISVCGZHLKNMSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Lutidine, also known as 2,6-dimethylpyridine, is an organic compound that is commonly used as a reagent in various organic reactions, such as the synthesis of heterocycles, nitroalkenes, and alkyl halides. It can also be used as a catalyst in organic synthesis.
Application
2,6-Lutidine can be used as:
- A base in the synthesis of an aldol adduct from malonic acid hemithioesters and aldehydes catalyzed by Cu(II) salt.
- An additive in reductive cyclization of epoxygeranyl acetate.
- A catalyst in combination with CuI for selective synthesis of N-sulfonyl-1,2,3-triazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
89.6 °F
Flash Point(C)
32 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cu (II)-catalyzed enantioselective aldol condensation between malonic acid hemithioesters and aldehydes.
Orlandi S, et al.
Tetrahedron Letters, 45(8), 1747-1749 (2004)
Copper-Catalyzed Synthesis of N-Sulfonyl-1, 2, 3-triazoles: Controlling Selectivity.
Yoo EJ, et al.
Angewandte Chemie (International Edition in English), 46(10), 1730-1733 (2007)
Ti (III)-catalyzed radical cyclization of 6, 7-epoxygeranyl acetate.
Fuse S, et al.
Tetrahedron Letters, 45(9), 1961-1963 (2004)
James R Frost et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(38), 13261-13277 (2015-08-01)
Since their isolation almost 20 years ago, the callipeltosides have been of long standing interest to the synthetic community owing to their unique structural features and inherent biological activity. Herein we present our full research effort that has led to the
Bojana Ginovska et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(44), 15713-15719 (2015-10-24)
We report that 2,6-lutidine⋅trichloroborane (Lut⋅BCl3 ) reacts with H2 in toluene, bromobenzene, dichloromethane, and Lut solvents producing the neutral hydride, Lut⋅BHCl2 . The mechanism was modeled with density functional theory, and energies of stationary states were calculated at the G3(MP2)B3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service