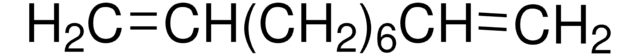All Photos(1)
About This Item
Linear Formula:
CH2=CH(CH2)10CH=CH2
CAS Number:
Molecular Weight:
194.36
Beilstein:
1741459
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
form
liquid
refractive index
n20/D 1.443 (lit.)
bp
131 °C/17 mmHg (lit.)
density
0.849 g/mL at 25 °C (lit.)
functional group
allyl
SMILES string
C=CCCCCCCCCCCC=C
InChI
1S/C14H26/c1-3-5-7-9-11-13-14-12-10-8-6-4-2/h3-4H,1-2,5-14H2
InChI key
XMRSTLBCBDIKFI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,13-Tetradecadiene is an α-diolefin. Polymerization of 1,13-tetradecadiene using aluminum triisobutyl-titanium tetrachloride was reported.
Application
1,13-Tetradecadiene was grafted to hydrogenated B-doped silicon (100) surfaces. It was used in the synthesis of naturally occurring, biologically active pyridine alkaloids theonelladins C and D, niphatesine C and xestamine D.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Monolayers of simple organic molecules on silicon studied by surface tools.
Scandurra A, et al.
Surface and Interface Analysis : SIA, 34(1), 777-777 (2002)
Polymerization of Higher a-Diolefins with Metal Alkyl Coordination Catalysts1.
Marvel CS and Garrison Jr WE.
Journal of the American Chemical Society, 81(17), 4737-4744 (1959)
Kotohiro Nomura et al.
Polymers, 12(1) (2019-12-22)
Copolymerizations of 1-decene (DC) with 1,9-decadiene (DCD), 1-dodecene (DD) with 1,11-dodecadiene (DDD), and 1-tetradecene (TD) with 1,13-tetradecadiene (TDD), using Cp*TiMe2(O-2,6-iPr2C6H3) (1)-[Ph3C][B(C6F5)4] (borate) catalyst in the presence of AliBu3/Al(n-C8H17)3 proceeded in a quasi-living manner in n-hexane at -30 to -50 °C
Synthesis of pyridine alkaloids via Pd-catalyzed coupling of 3-iodopyridine, 1, ?-dienes and nitrogen nucleophiles.
Larock RC and Wang Y.
Tetrahedron Letters, 43(1), 21-23 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service