All Photos(3)
About This Item
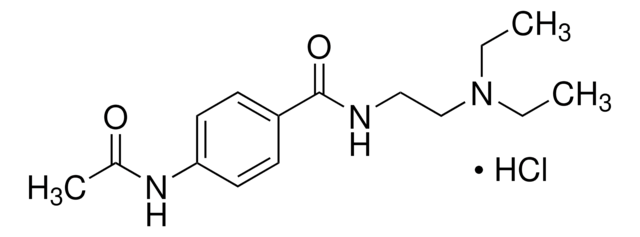
Linear Formula:
4-(CH3CONH)C6H4CONHCH2CH2N(C2H5)2
CAS Number:
Molecular Weight:
277.36
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
solid
mp
138-140 °C (lit.)
solubility
soluble 1%, clear, colorless to faintly yellow (1N HCl)
functional group
amide
amine
SMILES string
CCN(CC)CCNC(=O)c1ccc(NC(C)=O)cc1
InChI
1S/C15H23N3O2/c1-4-18(5-2)11-10-16-15(20)13-6-8-14(9-7-13)17-12(3)19/h6-9H,4-5,10-11H2,1-3H3,(H,16,20)(H,17,19)
InChI key
KEECCEWTUVWFCV-UHFFFAOYSA-N
General description
The relaxant effects of N-acetylprocainamide on bovine tracheal smooth muscle was studied.
Application
N-acetylprocainamide (NAPA) was used as a model drug in the study of establishing a quantitative approach to predict the renal clearances of basic drugs using N-1-methylnicotinamide (NMN).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S D Nelson et al.
The Journal of pharmacology and experimental therapeutics, 273(1), 315-319 (1995-04-01)
Ischemic zone refractoriness and conduction delay respond differently to infarct healing and, hypothetically, may exert discordant influences on the electrophysiologic action of different classes of antiarrhythmic drugs. This study evaluated the influence of infarct healing on the electrophysiologic effects of
J E Tisdale et al.
Therapeutic drug monitoring, 18(6), 693-697 (1996-12-01)
The objective of this study was to compare the precision and accuracy of fluorescence polarization immunoassay (FPIA) with high-performance liquid chromatography (HPLC) for measurement of procainamide (PA) and N-acetylprocainamide (NAPA) concentrations in urine. To determine the correlation between FPIA and
Multicenter evaluation of the Abbott AxSYM procainamide and N-acetylprocainamide assays: comparison with Abbott TDx/TDxFLx, Syva EMIT 2000, DuPont ACA, and HPLC methods.
H M Azzazy et al.
Clinical biochemistry, 31(1), 55-58 (1998-04-29)
Y L He et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 13(3), 303-308 (2001-06-01)
The dosage regimen of a drug eliminated predominantly through the kidney need to be adjusted for the patients with renal disease. The objective of the present study was to establish a quantitative approach to precisely predicting the renal clearances of
Application of capillary electrophoresis to the in vitro assessment of drug metabolism.
C M Hill et al.
Biochemical Society transactions, 23(3), 432S-432S (1995-08-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service