All Photos(1)
About This Item
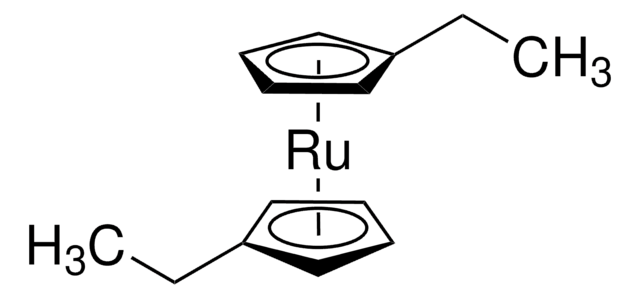
Empirical Formula (Hill Notation):
C10H10Ru
CAS Number:
Molecular Weight:
231.26
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
core: ruthenium
reagent type: catalyst
mp
199-201 °C (lit.)
SMILES string
[Ru].[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.Ru/c2*1-2-4-5-3-1;/h2*1-5H;
InChI key
BKEJVRMLCVMJLG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Malay Patra et al.
Chemical communications (Cambridge, England), 47(41), 11444-11446 (2011-09-22)
A convenient synthesis of azidomethyl-ruthenocene and its use in the covalent labelling of amino acids, peptides and a peptide nucleic acid (PNA) monomer derivative by Cu(I) catalyzed azide-alkyne coupling (Cu-AAC, "click chemistry") are described.
Cynthia T Sanderson et al.
Inorganic chemistry, 44(9), 3283-3289 (2005-04-26)
Electronic absorption and resonance Raman spectral studies of benzoylruthenocene (BRc) and 1,1'-dibenzoylruthenocene (DRc) indicate that the low-energy electronic excited states of these 4d(6) metallocenes possess metal-to-ligand charge transfer (MLCT) character. While this MLCT contribution should weaken the metal-ring bonding in
Results of ERBF and ERPF measurements in healthy dogs with two new radiopharmaceutical principles.
R de Jong et al.
Contributions to nephrology, 56, 49-52 (1987-01-01)
M Wenzel et al.
International journal of radiation applications and instrumentation. Part A, Applied radiation and isotopes, 37(6), 491-495 (1986-01-01)
The potential radiopharmaceuticals: ruthenocenoyl alanine, ruthenocenoyl methionine, 1'-methyl-ruthenocenoyl glycine and its esters were labelled with 103Ru starting from the analogous ferrocene compounds. In a series of tests in mice and rats these substances were compared with hippuran and ruppuran (=
M Wenzel et al.
Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft ... [et al], 162(1), 51-56 (1986-01-01)
The organ distribution of 103Ru labelled ruthenocenyl derivatives of tyramine, histamine, benzylamine, phenylethylamine and homoveratrylamine were measured in rats. The derivatives of tyramine, histamine and benzylamine showed a high affinity for the adrenal and ovar. Adrenal/muscle ratios up to 2000:1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service