262102
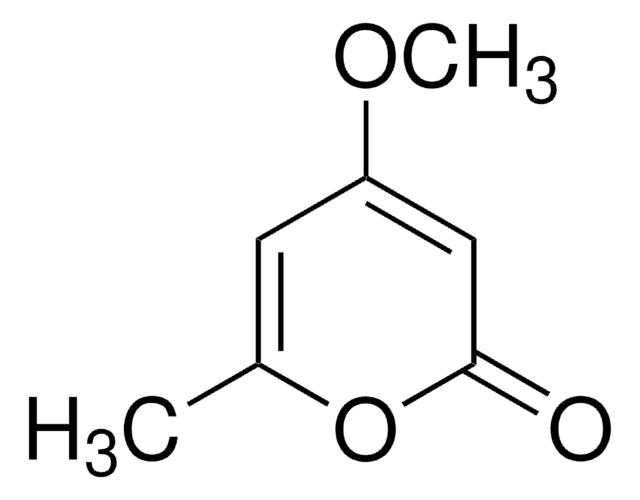
5,6-Dihydro-2H-pyran-2-one
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6O2
CAS Number:
Molecular Weight:
98.10
Beilstein:
1610
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
refractive index
n20/D 1.483 (lit.)
bp
103 °C/10 mmHg (lit.)
density
1.139 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
O=C1OCCC=C1
InChI
1S/C5H6O2/c6-5-3-1-2-4-7-5/h1,3H,2,4H2
InChI key
QBDAFARLDLCWAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Enantioselective conjugate addition of Grignard reagents to 5,6-dihydro-2H-pyran-2-one catalyzed by a chiral phosphine-copper iodide catalyst has been reported.
Application
5,6-Dihydro-2H-pyran-2-one has been used in the preparation of:
- (1aR,5aR,5bS,6S,7S)-6,7-di-tert-butoxy-5-oxo-pyrrolidino[1,2-b]isoxazolidino[4,5-c]tetrahydropyran
- 4-(phenyl)tetrahydro-2H-pyran-2-one
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
114.8 °F - closed cup
Flash Point(C)
46 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rhodium-catalyzed asymmetric 1, 4-addition of arylboron reagents to α,β-unsaturated esters.
Takaya Y, et al.
Tetrahedron Asymmetry, 10(20), 4047-4056 (1999)
D Socha et al.
Carbohydrate research, 336(4), 315-318 (2001-12-01)
(1aR,5aR,5bS,6S,7S)-6,7-Di-tert-butoxy-5-oxo-pyrrolidino[1,2-b]isoxazolidino[4,5-c]tetrahydropyran (8) prepared by (1,3)-dipolar cycloaddition of the cyclic nitrone 6 derived from tartaric acid to 5,6-dihydro-2H-pyran-2-one (7) was transformed into indolizidine 11 via a sequence of reactions involving methanolysis of the lactone ring, intramolecular alkylation of the nitrogen atom
Conjugate addition of diethylzinc to a, ?-unsaturated lactones catalyzed by copper-phosphite complexes.
Yan M, et al.
Chemical Communications (Cambridge, England), 2, 115-116 (2000)
Fabrizio Carta et al.
Bioorganic & medicinal chemistry letters, 22(1), 267-270 (2011-12-06)
The inhibition of the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1) with (thio)coumarins has been recently reported (Maresca et al., J. Am. Chem. Soc. 2009, 131, 3057). Here we demonstrate that a series of γ- and δ-(thio)lactones also act as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service