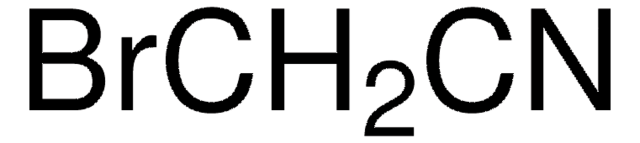257443
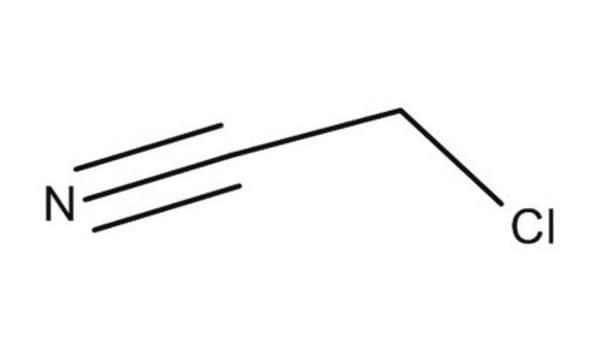
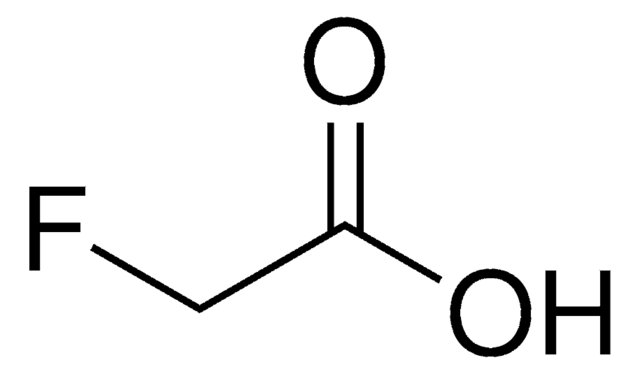
Fluoroacetonitrile
98%
Synonym(s):
2-Fluoroacetonitrile, Fluoromethyl cyanide, Monofluoroacetonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FCH2CN
CAS Number:
Molecular Weight:
59.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.333 (lit.)
bp
79-80 °C (lit.)
density
1.061 g/mL at 25 °C (lit.)
functional group
fluoro
nitrile
SMILES string
FCC#N
InChI
1S/C2H2FN/c3-1-2-4/h1H2
InChI key
GNFVFPBRMLIKIM-UHFFFAOYSA-N
Application
Fluoroacetonitrile has been used in preparation of:
- 2-fluoromethyl-4,4,6-trimethyl-1,3-oxazine
- α-fluorinated acetophenone
- 2-amino-2-fluoromethyl-3-pentenenitrile, a key intermediate in the synthesis of 2,5-diamino-2-fluoromethyl-3(E)-pentenoic acid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
6.8 °F - closed cup
Flash Point(C)
-14 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparative flow techniques. 2. Grignard addition reaction on fluoroaceto-nitrile: Synthesis of 2-amino-2-fluoromethyl-3-pentenenitrile.
Gerhart F, et al.
Journal of Fluorine Chemistry, 50(2), 243-249 (1990)
2-Fluoromethyl-4, 4, 6-trimetiiyl-1, 3-oxasine as a new reagent for the preparation of a-fluoroaldehydes.
Patrick TB, et al.
Tetrahedron Letters, 31(2), 179-182 (1990)
Erum Raja et al.
Tetrahedron letters, 52(40), 5170-5172 (2012-03-03)
Fluoro-substituted aliphatic nitriles are shown to undergo the Houben-Hoesch reactions with arenes in CF(3)SO(3)H to give fluoro-substituted ketones in good yields. The fluorine substituents appear to enhance the reactivities of the nitriles (and the nitrilium ion intermediates) compared to similar
Santanu Mondal et al.
ACS chemical biology, 13(4), 1057-1065 (2018-03-09)
Protein arginine deiminases (PADs) play an important role in the pathogenesis of various diseases, including rheumatoid arthritis, multiple sclerosis, lupus, ulcerative colitis, and breast cancer. Therefore, the development of PAD inhibitors has drawn significant research interest in recent years. Herein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service