232017
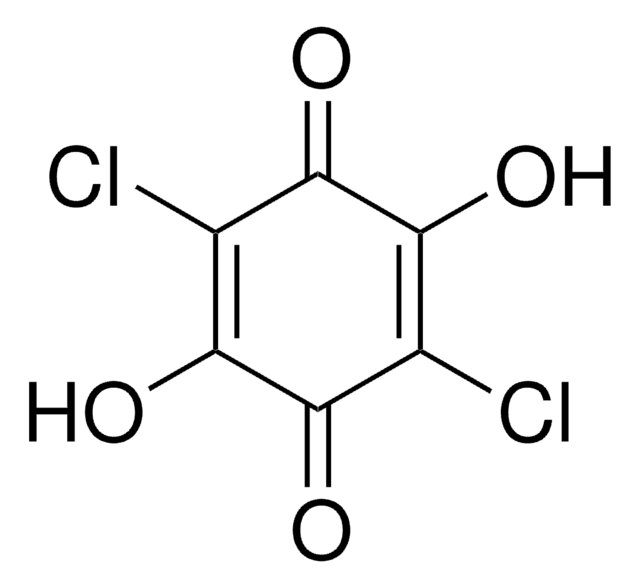
Tetrachloro-1,4-benzoquinone
99%
Synonym(s):
2,3,5,6-Tetrachloro-1,4-benzoquinone, p-Chloranil, Chloranil
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6Cl4(=O)2
CAS Number:
Molecular Weight:
245.88
Beilstein:
393006
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
solid
mp
289 °C (dec.) (lit.)
SMILES string
ClC1=C(Cl)C(=O)C(Cl)=C(Cl)C1=O
InChI
1S/C6Cl4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI key
UGNWTBMOAKPKBL-UHFFFAOYSA-N
Gene Information
human ... ACHE(43) , BCHE(590) , CES1(1066)
Related Categories
General description
Tetrachloro-1,4-benzoquinone (TCBQ) is a quinone compound with four chloride groups. The catalytic activity of quinone groups (benzoquinone) can be controlled by the chloride groups with large electronegativity.
Application
Synthesis of dibenzofurans via oxidative cyclization.
TCBQ can form a nanocomposite with multi-walled carbon nanotubes (MWCNTs) on a graphite electrode for nicotinamide adenine dinucleotide (NADH) oxidation. It can also provide pseudocapacitance and can be used as an electrode material for the development of supercapacitors. It may be used as an organic cathode and act as a redox mediator for the fabrication of lithium ion batteries.
Undergoes photoinduced cycloaddition reactions with stilbene derivatives and α,β-unsaturated carbonyl compounds. Fluorescence quencher.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of a polyacrylonitrile/tetrachloro-1, 4-benzoquinone gel polymer electrolyte for high-performance Li-air batteries.
Bok K, et al.
Journal of Membrane Science (2018)
Journal of the Chemical Society. Perkin Transactions 1, 571-571 (1994)
Tetsuya Takeya et al.
Organic letters, 9(15), 2807-2810 (2007-06-22)
A novel oxidative cyclization of quinone-arenols 5 leading to products 6 with a dibenzofuran-1,4-dione structure, which forms the core of several natural products, has been developed and applied to the synthesis of violet-quinone (4).
J. Prakt. Chem./Chem.-Ztg., 335, 515-515 (1993)
An Insoluble Benzoquinone-Based Organic Cathode for Use in Rechargeable Lithium-Ion Batteries.
Luo Z, et al.
Angewandte Chemie (International ed. in English), 56(41), 12561-12565 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service