226157
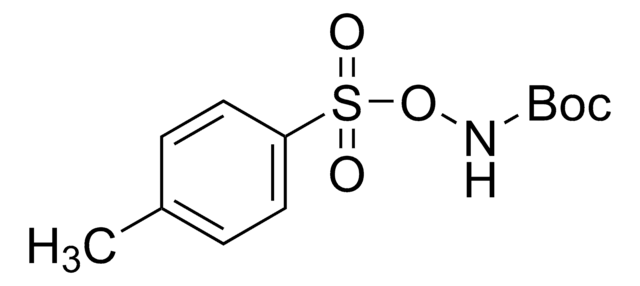
N-Boc-hydroxylamine
≥98%
Synonym(s):
tert-Butyl N-hydroxycarbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3COCONHOH
CAS Number:
Molecular Weight:
133.15
Beilstein:
1756546
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
solid
mp
53-55 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
CC(C)(C)OC(=O)NO
InChI
1S/C5H11NO3/c1-5(2,3)9-4(7)6-8/h8H,1-3H3,(H,6,7)
InChI key
DRDVJQOGFWAVLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Useful hydroxylamine derivatives for the synthesis of hydroxamic acids.
Tetrahedron Letters, 33(35), 5055-5058 (1992)
Tetrahedron Letters, 33, 5055-5055 (1992)
Synthetic Communications, 22, 2579-2579 (1992)
Brian S Bodnar et al.
The Journal of organic chemistry, 72(10), 3929-3932 (2007-04-14)
The addition of azides to acylnitroso hetero-Diels-Alder cycloadducts derived from cyclopentadiene affords exo-triazolines in excellent yield. The reaction is greatly affected by the level of alkene strain, while sterically demanding azides do not hinder the reaction. Conversion of the triazolines
M J Lee et al.
Journal of medicinal chemistry, 35(20), 3648-3652 (1992-10-02)
In the preceding paper, analogs of chlorpropamide with an OMe substituent on the sulfonamide nitrogen were shown to inhibit aldehyde dehydrogenase (AlDH), and it was postulated that these compounds were bioactivated by O-demethylation to release nitroxyl (HN = O, nitrosyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service