144088
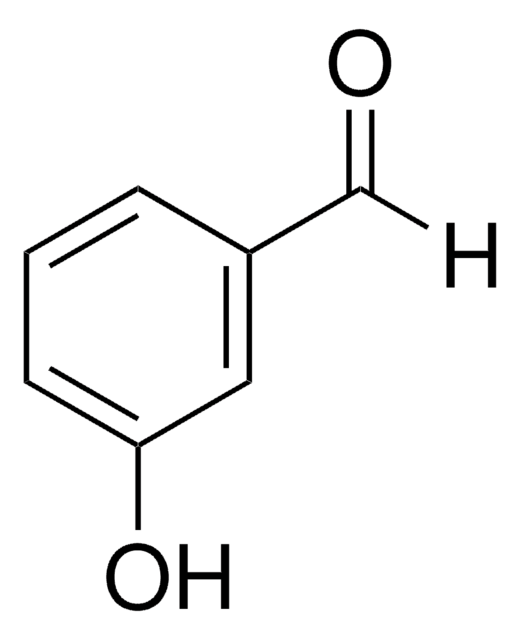
4-Hydroxybenzaldehyde
98%
Synonym(s):
4-Formylphenol, 4-Hydroxybenzaldehyde, Parahydroxybenzaldehyde, p-Formylphenol, p-Hydroxybenzaldehyde, p-Oxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4CHO
CAS Number:
Molecular Weight:
122.12
Beilstein:
471352
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
112-116 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc(O)cc1
InChI
1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
InChI key
RGHHSNMVTDWUBI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Hydroxybenzaldehyde can be used as a reactant to synthesize:
- 4-Hydroxybenzaldehydesemicarbazone by a condensation reaction with semicarbazide.
- Vanillin via bromination followed by copper-catalyzed coupling reaction with sodium methoxide.
- Oligo-4-hydroxybenzaldehyde by an oxidative polycondensation reaction with hydrogen peroxide (H2O2) in an alkaline medium.
- (−)-Centrolobine, a diarylheptanoid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
345.2 °F
Flash Point(C)
174 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The synthesis, characterization and thermal stability of oligo-4-hydroxybenzaldehyde
Mart H, et al.
Polymer Degradation and Stability, 83(3), 395-398 (2004)
Stereoselective synthesis of (-)-centrolobine
Lee Eun, et al.
Bulletin of the Korean Chemical Society,, 25(11), 1609-1610 (2004)
Vanillin synthesis from 4-hydroxybenzaldehyde
Taber Douglass F, et al.
Journal of Chemical Education, 84(7), 1158-1158 (2007)
Nadja Schultz-Jensen et al.
Applied biochemistry and biotechnology, 165(3-4), 1010-1023 (2011-07-06)
The potential of wheat straw for ethanol production after pretreatment with O(3) generated in a plasma at atmospheric pressure and room temperature followed by fermentation was investigated. We found that cellulose and hemicellulose remained unaltered after ozonisation and a subsequent
Hypomethylating agents (HMAs) effect on myelodysplastic/myeloproliferative neoplasm unclassifiable (MDS/MPN-U): single institution experience.
Aref Al-Kali et al.
Leukemia & lymphoma, 1-3 (2018-02-22)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service