All Photos(1)
About This Item
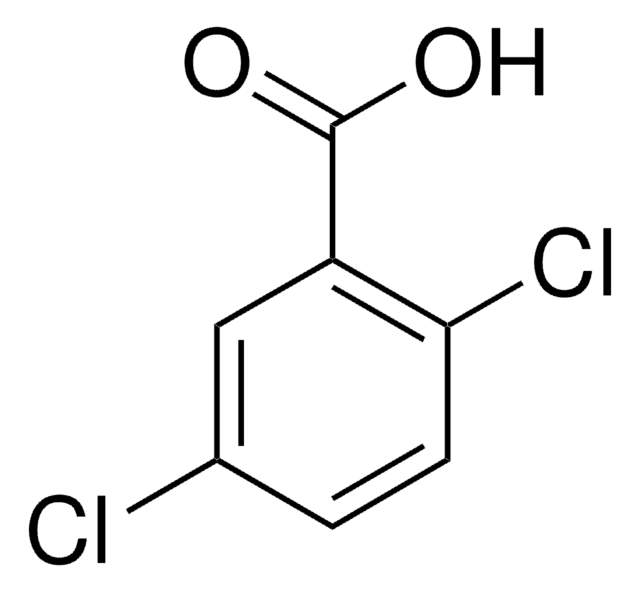
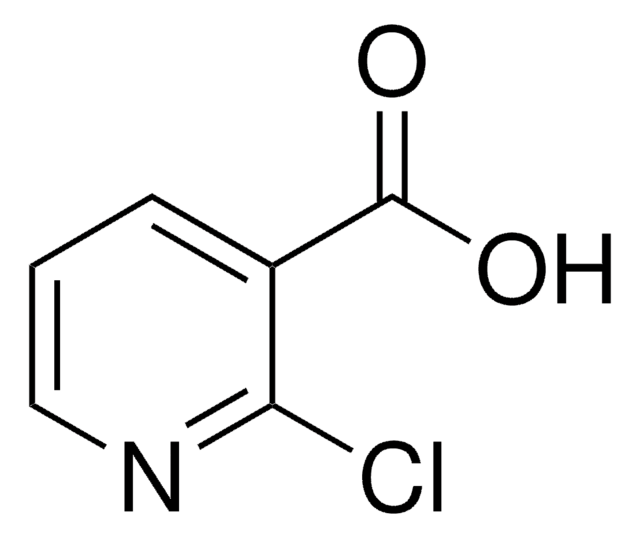
Linear Formula:
Cl2C6H3CO2H
CAS Number:
Molecular Weight:
191.01
Beilstein:
1868192
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
157-160 °C (lit.)
solubility
ethanol: soluble 1 g/10 mL, clear, colorless
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccc(Cl)cc1Cl
InChI
1S/C7H4Cl2O2/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3H,(H,10,11)
InChI key
ATCRIUVQKHMXSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dichlorobenzoic acid is the degradation product of chlorfenvinphos in water treated by photo-fenton driven by solar irradiation.
Application
2,4-Dichlorobenzoic acid was used in solid-phase extraction and gas chromatographic determination of polar acidic herbicides in surface water. It was used as reagent during the synthesis of pyrimido[2′,1′:2,3]thiazolo[4,5-b]quinoxaline derivatives. It was used as starting reagent during the synthesis of 1-(substituted)-1,4-dihydro-6-nitro-4-oxo-7-(sub-secondary amino)-quinoline-3-carboxylic acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gas chromatographic determination of acid herbicides in surface water samples with electron-capture detection and mass spectrometric confirmation.
Vink M and Van der Poll JM.
Journal of Chromatography A, 733(1), 361-366 (1996)
Umberto Costantino et al.
ACS applied materials & interfaces, 1(3), 668-677 (2010-04-02)
Benzoate (Bz), 2,4-dichlorobenzoate (BzDC), and p- and o-hydroxybenzoate (p- and o-BzOH) anions with antimicrobial activity have been intercalated into [Zn(0.65)Al(0.35)(OH)(2)](NO(3))(0.35).0.6H(2)O, layered double hydroxide (LDH), via anion-exchange reactions. The composition of the obtained intercalation compounds, determined by chemical, thermogravimetric, and ion
Anthony D Wright et al.
Chemosphere, 65(4), 604-608 (2006-03-24)
The toxic effects of several species of fresh water cyanobacteria, notably Microcystis species and associated toxins, the microcystins, Anabaena species (anatoxin), Nodularia sp. (nodularin), and Cylindrospermopsis raciborskii (cylindrospermopsin), are well known. Little, however, is known about the effects of secondary
Daniel J Repeta et al.
Environmental science & technology, 38(20), 5373-5378 (2004-11-17)
Chromophoric or colored dissolved organic matter (CDOM) is one of the principal light adsorbing components of seawater, particularly in the ultraviolet, where it attenuates over 90% of downwelling ultraviolet radiation. In highly productive coastal regions and throughout most of the
Sunday A Adebusoye et al.
Bioresource technology, 102(3), 3041-3048 (2010-11-16)
Multiple bacterial strains with CBA metabolic properties were isolated using a simple selective strategy. Phylogenetic analysis of the 16S rRNA gene sequences grouped them into two main clusters consisting of four bacterial phyla and belonging to 17 genera. Whereas growth
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service