131695
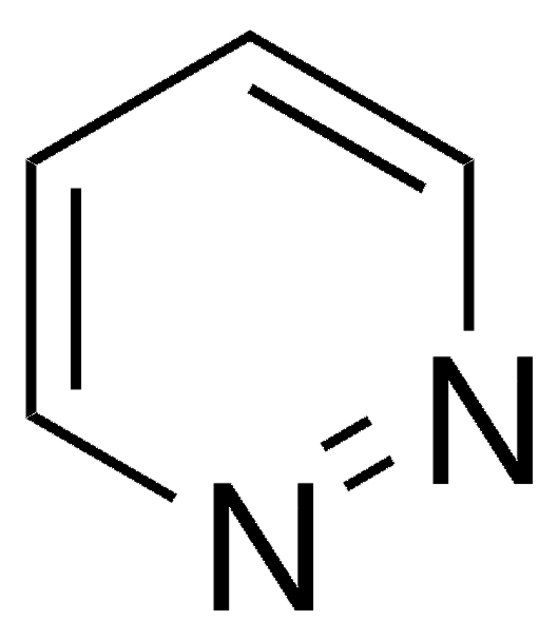
Pyrimidine
≥98.0%
Synonym(s):
1,3-Diazine, Metadiazine, m-Diazine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4N2
CAS Number:
Molecular Weight:
80.09
Beilstein:
103894
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39161701
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0%
refractive index
n20/D 1.504 (lit.)
bp
123-124 °C (lit.)
mp
19-22 °C (lit.)
solubility
H2O: soluble
alcohols: soluble
diethyl ether: soluble
density
1.016 g/mL at 25 °C (lit.)
SMILES string
c1cncnc1
InChI
1S/C4H4N2/c1-2-5-4-6-3-1/h1-4H
InChI key
CZPWVGJYEJSRLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyrimidine is a heterocyclic compound with excellent stability. It is used in manufacture of pharmaceuticals, agrochemicals and dyes.
Application
Pyrimidine was used to assess the extent of pyrimidine/purine asymmetry quantitatively. It was also used to study the photoinduced ion chemistry of the halogenated pyrimidines, a class of prototype radiosensitizing molecules.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
93.2 °F - closed cup
Flash Point(C)
34 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pyrimidine: a review on anticancer activity with key emphasis on SAR
Aastha M et al.
Future Journal of Pharmaceutical Sciences, 7, 123-123 (2021)
Y Vladimir Pabon-Martinez et al.
Scientific reports, 7(1), 11043-11043 (2017-09-10)
The anti-gene strategy is based on sequence-specific recognition of double-strand DNA by triplex forming (TFOs) or DNA strand invading oligonucleotides to modulate gene expression. To be efficient, the oligonucleotides (ONs) should target DNA selectively, with high affinity. Here we combined
Mattea Carmen Castrovilli et al.
Journal of the American Society for Mass Spectrometry, 25(3), 351-367 (2014-01-05)
In the present work, we studied the photoinduced ion chemistry of the halogenated pyrimidines, a class of prototype radiosensitizing molecules, in the energy region 9-15 eV. The work was stimulated by previous studies on inner shell site-selective fragmentation of the
R Piechocki et al.
TAG. Theoretical and applied genetics. Theoretische und angewandte Genetik, 49(6), 265-271 (1977-11-01)
The correlation between the evolutionary rates of proteins and frequencies of DNA-bases in the first and in the second position of corresponding codons was investigated.Adenine in the first and guanine in the second position showed the strongest positive correlation with
Ana A Fernández-Ramos et al.
Scientific reports, 7(1), 10550-10550 (2017-09-07)
Metabolic reprogramming is critical for T cell fate and polarization and is regulated by metabolic checkpoints, including Myc, HIF-1α, AMPK and mTORC1. Our objective was to determine the impact of mycophenolic acid (MPA) in comparison with rapamycin (Rapa), an inhibitor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service