All Photos(2)
About This Item
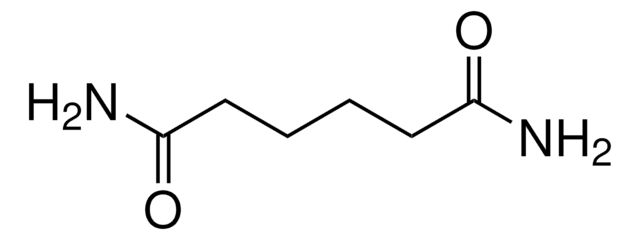
Linear Formula:
NH2COCH2CH2CONH2
CAS Number:
Molecular Weight:
116.12
Beilstein:
1753983
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
260-265 °C (dec.) (lit.)
solubility
cold water: soluble 220 part
boiling water: soluble 9 part
alcohol: insoluble
diethyl ether: insoluble
functional group
amide
SMILES string
NC(=O)CCC(N)=O
InChI
1S/C4H8N2O2/c5-3(7)1-2-4(6)8/h1-2H2,(H2,5,7)(H2,6,8)
InChI key
SNCZNSNPXMPCGN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Succinamide has been used in the characterization of a novel biuret hydrolase from the cysteine hydrolase superfamily. It was used as nitrogen supplement in the culture medium of Scenedesmus obliquus and green algae.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Watanabe et al.
Chemical & pharmaceutical bulletin, 45(9), 1458-1469 (1997-10-23)
A series of succinamide derivatives containing the 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one skeleton (6a-z) was prepared and evaluated for binding affinity to muscarinic receptors in vitro and for antagonism of bradycardia and salivation in vivo in comparison with AF-DX 116 (1a). Structure-activity relationships (SAR)
Petras P Dzeja et al.
American journal of physiology. Heart and circulatory physiology, 284(4), H1048-H1056 (2003-04-02)
Modulation of mitochondrial respiratory chain, dehydrogenase, and nucleotide-metabolizing enzyme activities is fundamental to cellular protection. Here, we demonstrate that the potassium channel opener diazoxide, within its cardioprotective concentration range, modulated the activity of flavin adenine dinucleotide-dependent succinate dehydrogenase with an
Birgit T Priest et al.
Biochemistry, 43(30), 9866-9876 (2004-07-28)
Sodium channel blockers are used clinically to treat a number of neuropathic pain conditions, but more potent and selective agents should improve on the therapeutic index of currently used drugs. In a high-throughput functional assay, a novel sodium channel (Na(V))
Elisabeth Garanger et al.
Bioconjugate chemistry, 20(1), 170-173 (2008-12-17)
The biotin/avidin system is one of the most widely used affinity detection and affinity capture systems in biology. However, the determination of the exact number of biotin tags attached onto a substrate is complicated by the fact that biotin does
Hideki Onagi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 9(24), 5978-5988 (2003-12-18)
Eight new [2]rotaxanes have been prepared, incorporating an alpha-cyclodextrin as the rotor, a stilbene as the axle, and trinitrophenyl substituents as capping groups. Strategies have been devised to elaborate these by linking the rotor to the axle, to produce two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service