116130
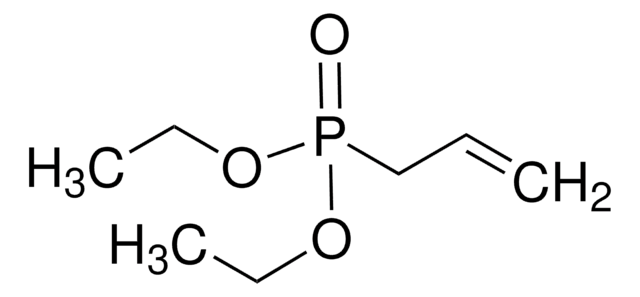
Diethyl vinylphosphonate
97%
Synonym(s):
Vinylphosphonic acid diethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHPO(OCH2CH3)2
CAS Number:
Molecular Weight:
164.14
Beilstein:
507596
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.429 (lit.)
bp
202 °C (lit.)
density
1.068 g/mL at 25 °C (lit.)
functional group
phosphonate
storage temp.
2-8°C
SMILES string
CCOP(=O)(OCC)C=C
InChI
1S/C6H13O3P/c1-4-8-10(7,6-3)9-5-2/h6H,3-5H2,1-2H3
InChI key
DREPONDJUKIQLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
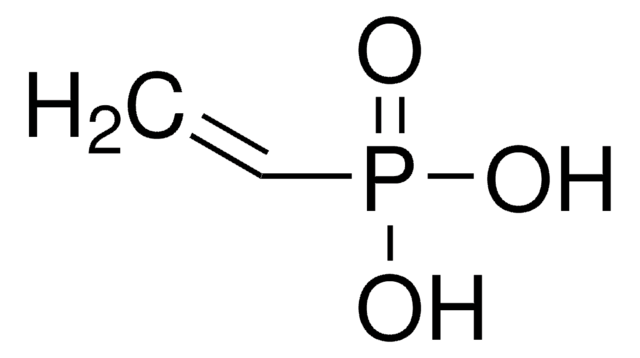
Diethyl vinylphosphonate (DEVP) can be used as a precursor for the synthesis of:
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
- α, β-unsaturated phosphonates by reacting with arylboronic acids via Pd-catalyzed Mizoroki−Heck reaction.
- 2-(arylamino)ethyl phosphonates by condensing with primary and secondary amines via the aza-Michael addition reaction.
It can be also employed as a monomer unit for the preparation of high-molecular-weight polymer, poly(diethyl vinylphosphonate) using lanthanide complexes.{18)
Diethyl vinylphosphonate has been used in the preparation of diethyl N-alkyl-2-aminoethylphosphonate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Convenient synthesis of α, β-unsaturated phosphonates via a Mizoroki-Heck reaction of arylboronic acids with diethyl vinylphosphonate
Kabalka GW, et al.
Tetrahedron Letters, 45(24), 4685-4687 (2004)
Synthesis of 2-(arylamino) ethyl phosphonic acids via the aza-Michael addition on diethyl vinylphosphonate
Orm Nadine B, et al.
Tetrahedron, 69(1), 115-121 (2013)
Poly (vinylphosphonate) s synthesized by trivalent cyclopentadienyl lanthanide-induced group transfer polymerization
Salzinger S, et al.
Macromolecules, 44(15), 5920-5927 (2011)
Joachim E Klee et al.
Beilstein journal of organic chemistry, 5, 72-72 (2009-01-01)
Novel N-alkyl-N-(phosphonoethyl) substituted mono-, bis- and tris(meth)acrylamides 3 were synthesized by two different three-step reactions and characterized by IR, (1)H NMR and (13)C NMR spectroscopy as well as refractive index and viscosity. The phosphonoethyl substituted (meth)acrylamide monomers show improved hydrolytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bis[2-(methacryloyloxy)ethyl] phosphate](/deepweb/assets/sigmaaldrich/product/structures/128/336/4e7a3e38-338c-423e-95b8-70d9d1f8e121/640/4e7a3e38-338c-423e-95b8-70d9d1f8e121.png)


