109274
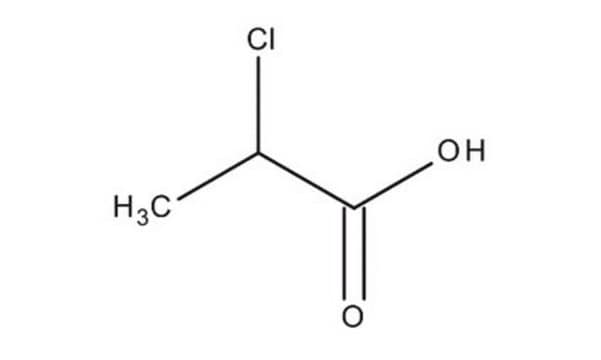
2-Chloropropionic acid
92%
Synonym(s):
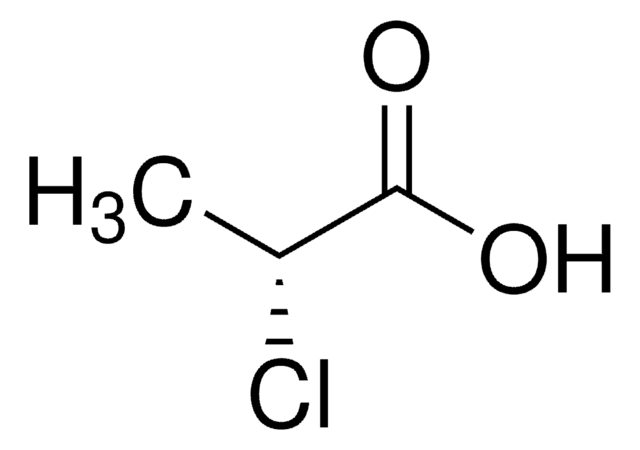
(±)-2-Chloropropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CHClCOOH
CAS Number:
Molecular Weight:
108.52
Beilstein:
1720259
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39050312
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
4 mmHg ( 20 °C)
Assay
92%
refractive index
n20/D 1.4345 (lit.)
bp
170-190 °C (lit.)
solubility
H2O: soluble
density
1.182 g/mL at 25 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
CC(Cl)C(O)=O
InChI
1S/C3H5ClO2/c1-2(4)3(5)6/h2H,1H3,(H,5,6)
InChI key
GAWAYYRQGQZKCR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(S)-2-Chloropropionic acid is the building block for the synthesis of aryloxyphenoxypropionic acid herbicides. 2-Chloropropionic acid is the raw material for production of pesticides, dyestuffs and agro- and forest chemicals.
Application
- 2-Chloropropionic acid is used in the preparation of propargyl 2-chloropropionate (PCP), an atom transfer radical polymerization (ATRP) initiator, by the esterification of propargyl alcohol.
- It can be employed in the synthesis of a biologically active chitin derivative, (1-carboxyethyl) chitosan.
- It can be treated with o-phenylenediamine phosphate to synthesize benzimidazole derivatives.
Biochem/physiol Actions
2-Chloropropionic acid on oral administration induces necrosis of granule cell layer of rat cerebellum.
Other Notes
Contains 2,2-dichloropropionic acid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
215.6 °F - closed cup
Flash Point(C)
102 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A facile and ?Green? synthesis of 2-substituted benzimidazoles.
Srinivas K and Dubey P K
Der Chemica Sinica, 5(2), 114-117 (2014)
Synthesis of a new chitin derivative,(1-carboxyethyl) chitosan.
Shigemasa Y, et al.
Chemistry Letters (Jpn), 24(8), 623-624 (1995)
Asymmetric reduction of 2-chloroacrylic acid to (S)-2-chloropropionic acid by a novel reductase from Burkholderia sp. WS.
Kurata A, et al.
Tetrahedron Asymmetry, 15(18), 2837-2839 (2004)
Synthesis of well-defined cyclic poly (N-isopropylacrylamide) via click chemistry and its unique thermal phase transition behavior.
Xu J, et al.
Macromolecules, 40(25), 9103-9110 (2007)
N C Sturgess et al.
Archives of toxicology, 74(3), 153-160 (2000-07-06)
L-2-Chloropropionic acid (L-CPA), when administered orally to rats, produces selective necrosis to the granule cell layer of the rat cerebellum which is delayed in onset, not appearing until 36-48 h after exposure. The present study was conducted to characterise the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service