104051
Picolinamide
98%
Synonym(s):
2-Pyridinecarboxamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O
CAS Number:
Molecular Weight:
122.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
110 °C (dec.) (lit.)
functional group
amide
SMILES string
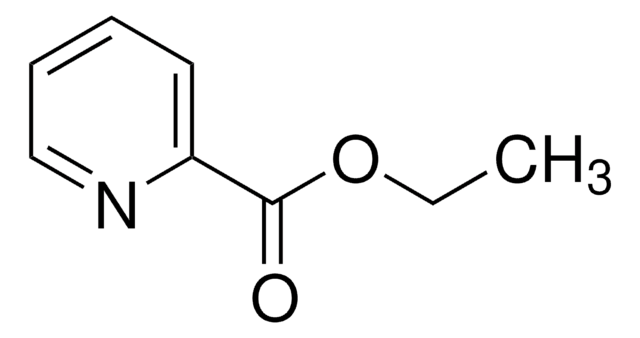
NC(=O)c1ccccn1
InChI
1S/C6H6N2O/c7-6(9)5-3-1-2-4-8-5/h1-4H,(H2,7,9)
InChI key
IBBMAWULFFBRKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Picolinamide was used as template in preparation of molecular imprinting polymer. Picolinamide was used in a study to evaluate kinetics and mechanism of liberation of picolinamide from chromium(III)-picolinamide complexes in HClO4.
Biochem/physiol Actions
Picolinamide is potential inhibitor of poly (ADP-ribose) synthetase of nuclei from rat pancreatic islet cells. Picolinamide acts as bidentate ligand and forms complexes with lanthanide nitrates, thiocyanates and perchlorates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Yamagami et al.
Cancer research, 45(4), 1845-1849 (1985-04-01)
Streptozotocin and alloxan were administered to Wistar rats in combination with poly(adenosine diphosphate ribose) synthetase inhibitors. Ten to 16 months after the injection of streptozotocin (50 mg/kg body weight i.v.) and 3-aminobenzamide (345 mg/kg i.v.), streptozotocin (50 mg/kg) and nicotinamide
New chromium (III)-picolinamide complexes. Kinetics and mechanism of picolinamide liberation in HClO4 solutions.
Pazderska-Szablowicz M, et al.
Transition Met. Chem. (London), 31(8), 1075-1080 (2006)
Ryan A Olsen et al.
Journal of the American Chemical Society, 125(33), 10125-10132 (2003-08-14)
Pyridine carboxamides are a class of medicinal agents with activity that includes the reduction of iron-induced renal damage, the regulation of nicotinamidase activity, and radio- and chemosensitization. Such pharmacological activities, and the prevalence of the carboxamide moiety and the importance
John N Zvimba et al.
Journal of inorganic biochemistry, 101(8), 1120-1128 (2007-06-15)
The protonation equilibria of a pentadentate ligand, N,N'-(2,2'-azanediylbis(ethane-2,1-diyl))dipicolinamide ([H(2)(5555)-N]) and the complexation of this ligand with Cu(II) Ca(II), Zn(II) and Ni(II) have been studied by pH-potentiometry, (1)H NMR spectroscopy and UV-vis spectrophotometry. (1)H NMR detected the protonation of the pyridyl
T Kawabata et al.
Acta pathologica japonica, 42(7), 469-475 (1992-07-01)
The effects of three isomers of pyridinecarboxamide (picolinamide (2-pyridinecarboxamide), nicotinamide (3-pyridinecarboxamide) and isonicotinamide (4-pyridinecarboxamide)) on iron-induced renal damage were studied. Pyridinecarboxamide (250 mg/kg body weight, ip) was administered 10 min before injection of ferric nitrilotriacetate (Fe(III)-NTA) (7.5 mgFe/kg body weight
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service