101915
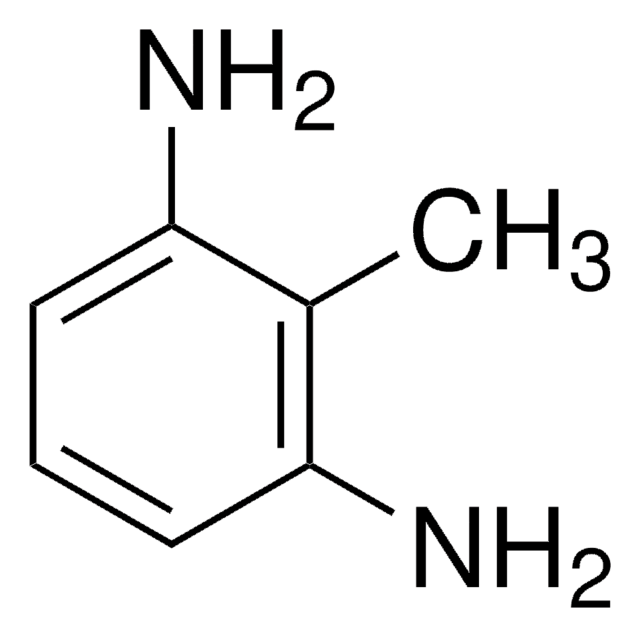
2,4-Diaminotoluene
98%
Synonym(s):
4-Methyl-m-phenylenediamine, 2,4-Diaminotoluene, 2,4-Toluenediamine, 4-Methyl-1,3-phenylenediamine
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
bp
283-285 °C (lit.)
mp
97-99 °C (lit.)
SMILES string
Cc1ccc(N)cc1N
InChI
1S/C7H10N2/c1-5-2-3-6(8)4-7(5)9/h2-4H,8-9H2,1H3
InChI key
VOZKAJLKRJDJLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Employing UV/H2O2 process for degradation of 2, 4-Diaminotoluene in synthetic wastewater: This study explores the degradation of carcinogenic Toluene-2, 4-diamine in synthetic wastewater using the UV/H2O2 process, highlighting its potential for improving water treatment methods (J Hosseini, A Shokri, 2017).
- Development of magnetic, ferrite supported palladium catalysts for 2, 4-dinitrotoluene hydrogenation: The paper discusses the use of Pd/NiFe2O4 catalyst for efficient hydrogenation of 2,4-dinitrotoluene to 2,4-diaminotoluene, demonstrating a high yield and potential for industrial applications (V Hajdu, M Varga, G Muránszky, G Karacs, 2021).
- Synthesis, characterisation and energetic performance of insensitive energetic salts formed between picric acid and 2, 3-diaminotoluene, 2, 4-diaminotoluene: This paper presents the synthesis and characterization of new energetic materials, which could be significant for the development of safer explosives and pyrotechnic devices (N Şen, H Nazir, N Atҫeken, KS Hope, N Acar, 2020).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 1B - Muta. 2 - Repr. 2 - Skin Sens. 1 - STOT RE 2
Target Organs
Liver,Kidney
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service