All Photos(2)
About This Item
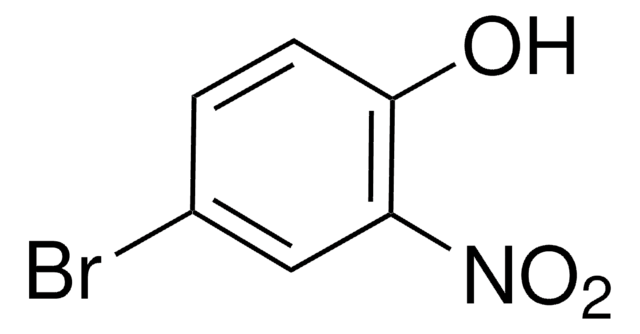
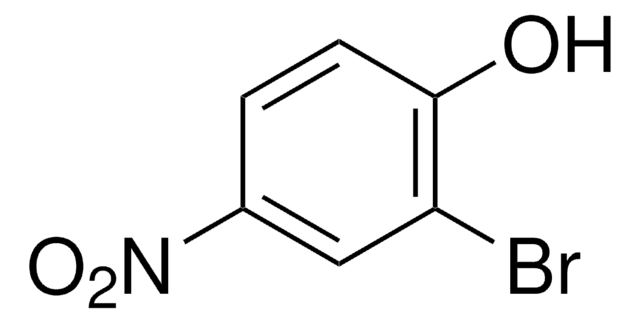
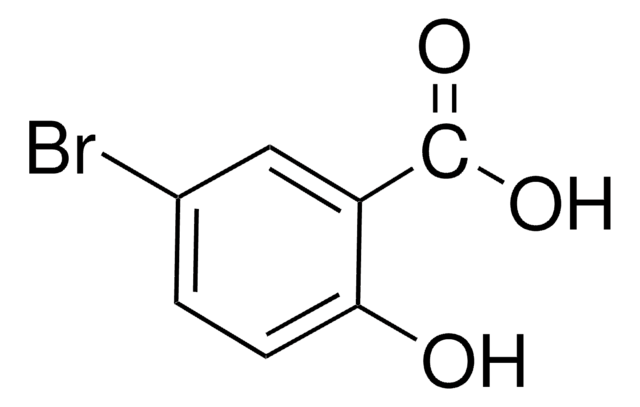
Linear Formula:
BrC6H4OH
CAS Number:
Molecular Weight:
173.01
Beilstein:
1853950
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
236 °C (lit.)
mp
28-32 °C (lit.)
functional group
bromo
SMILES string
Oc1cccc(Br)c1
InChI
1S/C6H5BrO/c7-5-2-1-3-6(8)4-5/h1-4,8H
InChI key
MNOJRWOWILAHAV-UHFFFAOYSA-N
Gene Information
human ... ALOX12(239) , ALOX15(246)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Bromophenol is used for suzuki-miyaura coupling reaction and in the synthesis of pentacyclic building block benzodibenzofuranquinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of a versatile pentacyclic building block for organic electronics
Brenden M et al.
Journal of Polymer Science Part A: Polymer Chemistry, 55, 2618-2628 (2017)
Palladium loaded on ZnO nanoparticles: Synthesis, characterization and application as heterogeneous catalyst for Suzuki--Miyaura cross-coupling reactions under ambient and ligand-free conditions
Digambar B B et al.
Materials Chemistry and Physics, 243, 122561-122561 (2020)
Kesong Tian et al.
Nature communications, 11(1), 3884-3884 (2020-08-06)
Integrating nitrogen species into sp2-hybridized carbon materials has proved an efficient means to improve their electrochemical performance. Nevertheless, an inevitable mixture of nitrogen species in carbon materials, due to the uncontrolled conversion among different nitrogen configurations involved in synthesizing nitrogen-doped
D Mahadevan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 78(2), 575-581 (2010-12-28)
The FT-IR and FT-Raman spectra of 3-Bromo phenol (3-BP) molecule have been recorded using Bruker IFS 66V spectrometer in the range of 4000-100 cm(-1). The molecular geometry and vibrational frequencies in the ground state are calculated by using the ab
G F Rush et al.
Toxicology, 30(3), 259-272 (1984-04-02)
Bromobenzene, at doses greater than 5.7 mmol/kg, produced renal proximal tubular necrosis and renal functional changes in mice. p-Bromophenol and o-bromophenol were the major urinary phenolic bromobenzene metabolites although m-bromophenol and 4-bromocatechol were also excreted in detectable quantities. With the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service