SAE0090
Beta-galactoside alpha-2,6-sialyltransferase 1
≥300 units/mg protein, ST6GAL1 human recombinant, expressed in HEK 293 cells
Synonym(s):
Alpha 2,6-ST 1, B-cell antigen CD75, CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,6-sialyltransferase 1, ST6Gal I, Sialyltransferase 1
About This Item
Recommended Products
recombinant
expressed in HEK 293 cells
description
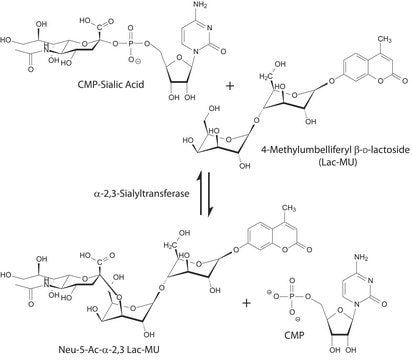
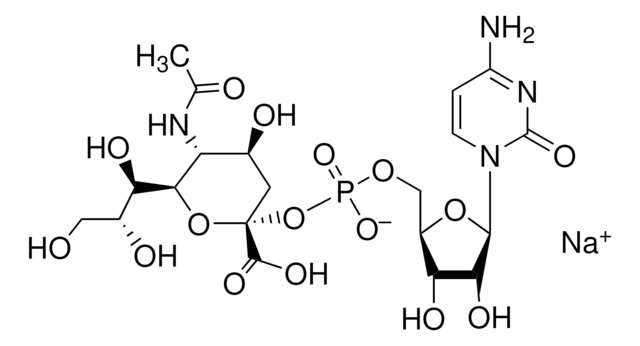
The specific activity of ST6Gal I is measured by its ability to transfer sialic acid from CMP-NANA to asialofetuin.
Assay
≥95% (SDS-PAGE)
form
lyophilized powder
specific activity
≥300 units/mg protein
shipped in
ambient
storage temp.
−20°C
General description
Biochem/physiol Actions
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity,3 as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.4-5
This recombinant ST6Gal I product can be used to study the mode of action of the enzyme, as well as its potential inhibitors. It can also be used as a glycoengineering tool to modify glycoproteins in vitro.
CMP-N-acetylneuraminate (CMP-sialic acid, CMP-NANA) to the β-D-galactosyl-1,4-N-acetyl-D-glucosaminyl termini on glycoproteins.
Sialic acids are distributed in a variety of glycolipids and glycoproteins. The sialic acid that is added to a galactose (Gal) can be bound either to the hydroxyl attached to carbon-3 of Gal to form an α-2,3 glycosidic linkage, or to the hydroxyl group attached to carbon-6 to form an α-2,6 glycosidic linkage. ST6Gal I generates a α-2,6 linkage of sialic acid on the non-reducing, terminal Galβ1 4GlcNAc residues of oligosaccharides and glycoconjugates.
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity, as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.
Unit Definition
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service