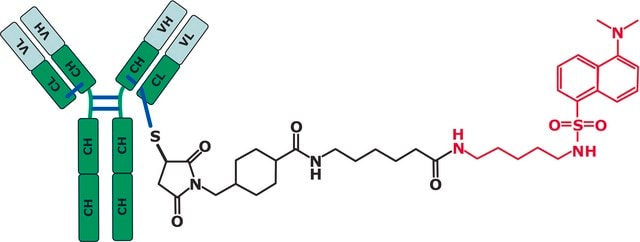
MSQC3
SILu™MAB Stable-Isotope Labeled Universal Monoclonal Antibody Standard human
recombinant, expressed in CHO cells
Synonym(s):
Mass spectrometry standard, IgG, Mass spectrometry standard, Immunoglobulin-G, Stable isotope labelled IgG
About This Item
Recommended Products
recombinant
expressed in CHO cells
Quality Level
Assay
≥90% (SDS-PAGE)
packaging
vial of 100 μg (± 10% Lot-specific vial content given on certificate of analysis)
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
DTLMISRHeavy Chain (IgG1, IgG2, IgG3, IgG4)
FNWYVDGVEVHNAKHeavy Chain (IgG1)
VVSVLTVLHQDWLNGKHeavy Chain (IgG1, IgG3, IgG4)
NQVSLTCLVKHeavy Chain (IgG1, IgG2, IgG3, IgG4)
GFYPSDIAVEWESNGQPENNYKHeavy Chain (IgG1, IgG4)
AGVETTTPSKLight Chain (lambda)
YAASSYLSLTPEQWKLight Chain (lambda)
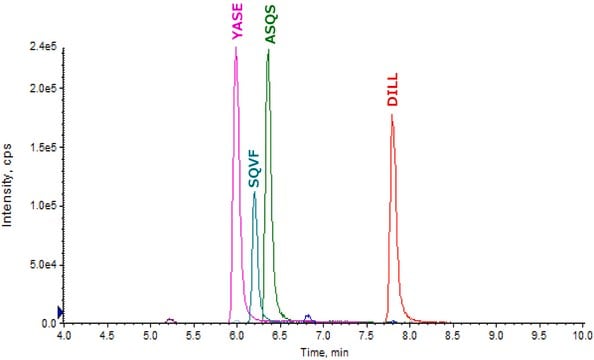
- SILuMab yielded reproducible, linear curves from 0.1 μg/mL to 1000 μg/mL without enrichment or depletion.
- Good agreement was observed between multiple peptides derived from the same target.
- Label incorporation was determined to be >98% by mass spectrometry.
- Sequence coverage was confirmed by peptide mapping.
Physical form
Preparation Note
Reconstitution
Procedure
- Briefly centrifuge the vial at ~10,000 x g to collect the product at the bottom of the vial.
- Add 500 μL of purified water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for a minimum of 15 minutes and repeat mixing by inversion.
Analysis Note
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SILuMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
Target overlap areas are underlined
Intact heavy and light chains (FASTA file)
Quantitative
MRM settings provided (Skyline, xls)
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover our wide variety of products for intact mass analysis of monoclonal antibodies, including size-exclusion columns (SEC), ion exchange columns, reverse-phase columns, HPLC buffers, MALDI matrices and standards, high-purity solvents, reagents, tools for protein sample preparation, and certified reference materials.
Discover our wide variety of products for intact mass analysis of monoclonal antibodies, including size-exclusion columns (SEC), ion exchange columns, reverse-phase columns, HPLC buffers, MALDI matrices and standards, high-purity solvents, reagents, tools for protein sample preparation, and certified reference materials.
Discover our wide variety of products for intact mass analysis of monoclonal antibodies, including size-exclusion columns (SEC), ion exchange columns, reverse-phase columns, HPLC buffers, MALDI matrices and standards, high-purity solvents, reagents, tools for protein sample preparation, and certified reference materials.
Discover our wide variety of products for intact mass analysis of monoclonal antibodies, including size-exclusion columns (SEC), ion exchange columns, reverse-phase columns, HPLC buffers, MALDI matrices and standards, high-purity solvents, reagents, tools for protein sample preparation, and certified reference materials.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service