163031
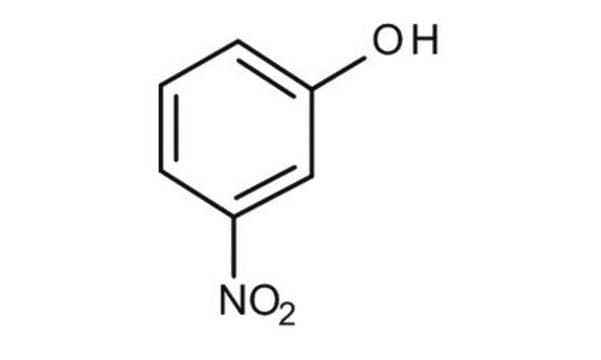
3-Nitrophenol
ReagentPlus®, 99%
Synonym(s):
3-NP, m-NP, m-Nitrophenol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4OH
CAS Number:
Molecular Weight:
139.11
Beilstein:
1907946
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
194 °C/70 mmHg (lit.)
mp
96-98 °C (lit.)
SMILES string
Oc1cccc(c1)[N+]([O-])=O
InChI
1S/C6H5NO3/c8-6-3-1-2-5(4-6)7(9)10/h1-4,8H
InChI key
RTZZCYNQPHTPPL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Nitrophenol (m-nitrophenol) is a nitroaromatic compound. It can be prepared from 3-nitroaniline, via diazotization reaction.
3-Nitrophenol is one of the isomers of mononitrophenol and is mainly used as an intermediate to prepare dyes, pigments, lumber preservatives, photographic chemicals and pesticides. Some of the methods for its degradation are biotransformation, photocatalytic degradation and photooxidation.
Application
3-Nitrophenol may be used in the preparation of 3-aminophenol. It may be employed as a weak acid in capillary isoelectric focusing (cIEF) method.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways.
Haigler BE and Spain JC.
Applied and Environmental Microbiology, 57(11), 3156-3162 (1991)
Risky Ayu Kristanti et al.
Environmental science and pollution research international, 19(5), 1852-1858 (2012-03-08)
The accelerated biodegradation of 3-nitrophenol (3-NP) in the rhizosphere of giant duckweed (Spirodela polyrrhiza) was investigated. Biodegradation of 3-nitrophenol in the rhizosphere of a floating aquatic plant, S. polyrrhiza, was investigated by using three river water samples supplemented with 10
Eagleson M.
Concise Encyclopedia Chemistry, 700-700 (1994)
Purnendu K Dasgupta
Journal of chromatography. A, 1213(1), 50-55 (2008-09-16)
Resolution of overlapped chromatographic peaks is generally accomplished by modeling the peaks as Gaussian or modified Gaussian functions. It is possible, even preferable, to use actual single analyte input responses for this purpose and a nonlinear least squares minimization routine
Photocatalytic degradation of nitrobenzenes with combustion synthesized nano-TiO 2.
Priya MHl and Madras G.
Journal of Photochemistry and Photobiology A: Chemistry, 178(1), 1-7 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service