917060
1 M Biphenyl in DME
a precursor solution to prepare the prelithiation/presodiation reagent for disordered carbon, SiO, Sn, Sb, P, and S electrodes
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
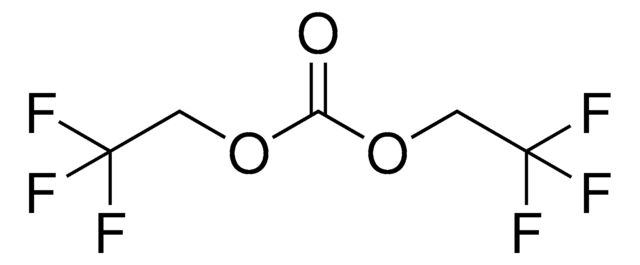
C12H10
Molecular Weight:
154.21
MDL number:
UNSPSC Code:
12352401
NACRES:
NA.23
Recommended Products
Related Categories
Application
This product is biphenyl (Bp) solution in anhydrous DME (1 mol/L) is a precursor reagent that could be easily handled to prepare prelithiation or presodiation reagent. By direct reaction of Li metal, it forms a strong reducing reagent of Li-Bp. The resulting Li-Bp/DME reagent is applicable to prelithiate most of the active materials for Li ion batteries, such as Sn, Sb, P, S, SiO and other metal oxide. On the other hand, a presodiation reagent can be prepared by reacting Bp/DME solution with Na metal. Na-Bp/DME reagent can be used to presodiate hard carbon, a promising anode material in Na ion batteries.
Preparation Note
Suggested Directions for use:
To prepare 1 M Li-Bp/DME reagent, > 0.07 g of Li metal is added into every 10 ml of 1 M BP/DME solution.
To prepare 1 M Na-Bp/DME reagent, > 0.23 g of Na metal is added into every 10 ml of 1 M BP/DME solution.
- Step 1 Preparation of Prelithiation/presodiation reagent
To prepare 1 M Li-Bp/DME reagent, > 0.07 g of Li metal is added into every 10 ml of 1 M BP/DME solution.
To prepare 1 M Na-Bp/DME reagent, > 0.23 g of Na metal is added into every 10 ml of 1 M BP/DME solution.
- Step 2 Prelithiation/presodiation of electrodes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Eye Irrit. 2 - Flam. Liq. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
33.8 °F
Flash Point(C)
1 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High performance lithium-ion and lithium−sulfur batteries using prelithiated phosphorus/carbon composite anode..
Wang F, et al.
Energy Storage Materials, 24, 147-152 (2020)
High performance lithium-ion and lithium?sulfur batteries using prelithiated phosphorus/carbon composite anode.
Wang G, et al.
Energy Storage Materials, 24, 147-152 (2020)
Gongwei Wang et al.
ACS applied materials & interfaces, 11(9), 8699-8703 (2019-02-20)
This study reports an ambient-air-tolerant approach for negative electrode prelithiation by using 1 M lithium-biphenyl (Li-Bp)/tetrahydrofuran (THF) solution as the prelithiation reagent. Key to this strategy are the relatively stable nature of 1 M Li-Bp/THF in ambient air and the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service