528986
2-Fluoro-4-methoxybenzaldehyde
97%
Synonym(s):
2-Fluoro-p-anisaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
154.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
43-48 °C (lit.)
storage temp.
2-8°C
SMILES string
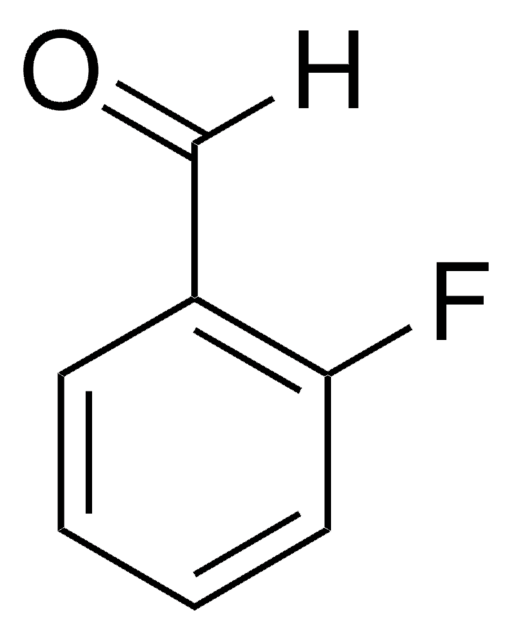
COc1ccc(C=O)c(F)c1
InChI
1S/C8H7FO2/c1-11-7-3-2-6(5-10)8(9)4-7/h2-5H,1H3
InChI key
UNWQNFJBBWXFBG-UHFFFAOYSA-N
General description
2-Fluoro-4-methoxybenzaldehyde is a fluorinated aromatic aldehyde. It can be prepared from 4-bromo-3-fluoroanisole.
Application
2-Fluoro-4-methoxybenzaldehyde may be used in the preparation of:
- fluorine containing 2,4,5-trisubstituted imidazole
- 1-(2-fluoro-4-methoxyphenyl)-2-propanone
- 6-(2-fluoro-4-methoxyphenyl)fulvene
- 10-(2-fluoro-4-methoxyphenyl)-6,7,9,10-tetrahydro-1Hfuro[3,4-b]pyrazolo[3,4-f]quinolin-9-one
- polyhydroquinoline (PHQ)
- 3-(2-fluoro-4-methoxyphenyl) acrylic acid methyl ester
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fluorinated derivatives of titanocene Y: synthesis and cytotoxicity studies.
Claffey J, et al.
European Journal of Organic Chemistry, 26, 4074-4082 (2008)
Synthesis of 1, 3-Bis (hydroxy-halogenophenyl)-propane-1, 3-diamines and their Pt (II) Complexes, Syntheses of the Ligands.
Kammermeier T and Wiegrebe W.
Arch. Pharm. (Weinheim), 327, 547-561 (1994)
Indium trifluoride: A highly efficient catalyst for the synthesis of fluorine-containing 2, 4, 5-trisubstituted imidazoles under solvent-free conditions.
Reddy MV and Jeong YT.
Journal of Fluorine Chemistry, 142, 45-51 (2012)
Polystyrene-Supported p-Toluenesulfonic Acid: A New, Highly Efficient, and Recyclable Catalyst for the Synthesis of Hydropyridine Derivatives under Solvent-Free Conditions.
Reddy MV and Jeong YT.
Synlett, 23(20), 2985-2991 (2012)
Synthesis, cytotoxic activity and docking studies of new 4-aza-podophyllotoxin derivatives.
Hatti I, et al.
Medicinal Chemistry Research, 24(8), 3305-3313 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service