497312
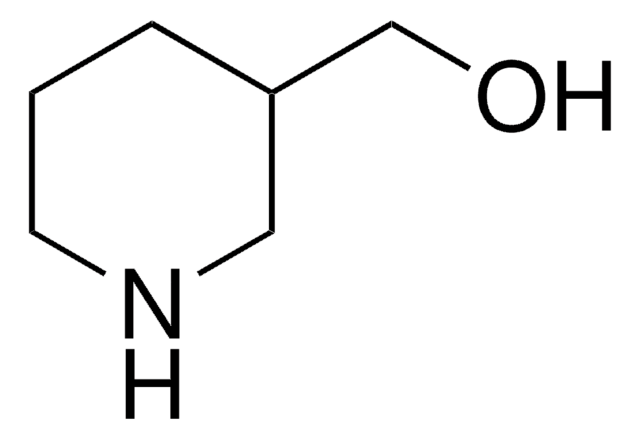
4-Piperidinemethanol
97%
Synonym(s):
4-(Hydroxymethyl)piperidine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H13NO
CAS Number:
Molecular Weight:
115.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
bp
118-120 °C/10 mmHg (lit.)
mp
55-59 °C (lit.)
SMILES string
OCC1CCNCC1
InChI
1S/C6H13NO/c8-5-6-1-3-7-4-2-6/h6-8H,1-5H2
InChI key
XBXHCBLBYQEYTI-UHFFFAOYSA-N
Related Categories
General description
4-Piperidinemethanol is a cyclic secondary amine. Its standard molar enthalpies of combustion, sublimation and formation have been determined.
Application
4-Piperidinemethanol may be used in the preparation of:
- N-tert-butoxycarbonyl-4-hydroxymethyl piperidine
- desferrioxamine B (DFO) containing third generation triazine dendrimer
- ethyl 3-(4-(hydroxymethyl)piperidin-1-yl)propanoate (EHMPP)
- 4-(hydroxymethyl)piperidine-1-carbodithioic acid (HL)
- 1-[[(1E)-2-(4-chlorophenyl)ethenyl]sulfonyl]-4-piperidinemethanol
Substrate used in solid-phase organic synthesis of a secondary amine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christian A Olsen et al.
Organic letters, 6(12), 1935-1938 (2004-06-05)
[reaction: see text] An expedient solid-phase synthetic approach to secondary and tertiary amines was developed. The protocol employs conversion of resin-bound amino alcohols to the corresponding iodides, followed by iodide displacement with primary or secondary amines or with unprotected amino
Synthesis, spectroscopy, and biological activity of heterobimetallic complexes containing Sn (IV) and Pd (II) with 4-(hydroxymethyl) piperidine-1-carbodithioic acid.
Anwar MT, et al.
Russ. J. Gen. Chem., 83(12), 2380-2385 (2013)
Standard molar enthalpies of formation of 2-, 3-, and 4-piperidinomethanol isomers.
da Silva MAVR and Cabral JITA
The Journal of Chemical Thermodynamics, 38(8), 1008-1012 (2006)
Jongdoo Lim et al.
Bioorganic & medicinal chemistry, 18(15), 5749-5753 (2010-07-10)
The synthesis of a third generation triazine dendrimer, 1, containing multiple, iron-sequestering desferrioxamine B (DFO) groups is described. Benzoylation of the hydroxamic acid groups of DFO and formation of a reactive dichlorotriazine provide the intermediate for reaction with the second
Enzymatic synthesis, thermal and crystalline properties of a poly (β-amino ester) and poly (lactone-co-β-amino ester) copolymers.
Martino L, et al.
Polymer, 53(9), 1839-1848 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)



