All Photos(1)
About This Item
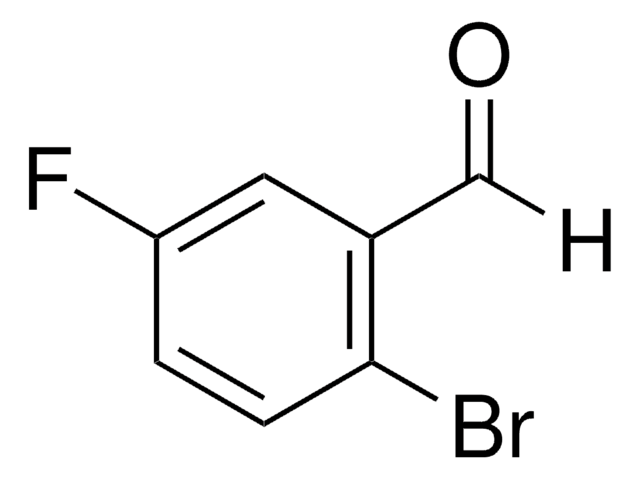
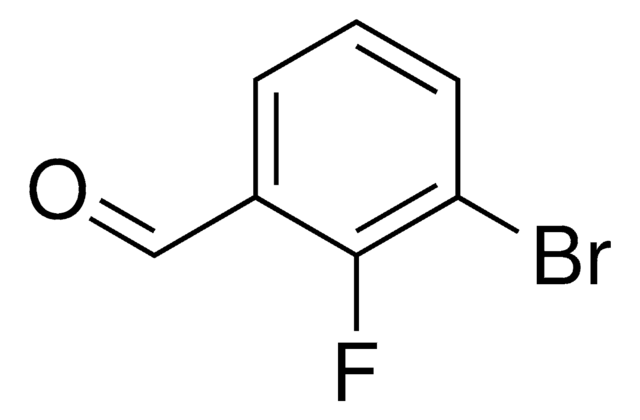
Linear Formula:
BrC6H3(F)CHO
CAS Number:
Molecular Weight:
203.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
96%
form
solid
mp
58-62 °C (lit.)
SMILES string
Fc1cc(Br)ccc1C=O
InChI
1S/C7H4BrFO/c8-6-2-1-5(4-10)7(9)3-6/h1-4H
InChI key
UPCARQPLANFGQJ-UHFFFAOYSA-N
Application
4-Bromo-2-fluorobenzaldehyde has been used in the preparation of:
- 2-functionalized aromatic monoaldehydes, via reaction with different secondary amines and phenol
- fluorostilbenes
- benzyl amine-based histamine H3 antagonist having serotonin reuptake activity
- 6-bromo-2-(4-bromo-2-fluorophenyl)-2,3-dihydro-4H-chromen-4-one
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Richard J Sciotti et al.
Bioorganic & medicinal chemistry letters, 12(16), 2121-2123 (2002-07-20)
A novel series of antimicrobials of the oxazolidinone class is disclosed. These compounds are characterized relative to previously described analogues by a 'halostilbene-derived' pharmacophore and demonstrate enhanced antimicrobial activity against key Gram-positive pathogens when compared to Linezolid.
Michael A Letavic et al.
Bioorganic & medicinal chemistry letters, 17(17), 4799-4803 (2007-07-10)
The design, synthesis, and in vitro activity of a series of novel 5-ethynyl-2-aryloxybenzylamine-based histamine H(3) ligands that are also serotonin reuptake transporters is described.
Hany F Nour et al.
Organic & biomolecular chemistry, 9(9), 3258-3271 (2011-03-25)
2-Functionalised aromatic monoaldehydes were synthesised in good to excellent yields by reacting 4-bromo-2-fluorobenzaldehyde with different secondary amines and phenol. The Suzuki-coupling reaction of the newly functionalised aromatic monoaldehydes with 4-formylphenylboronic acid afforded the corresponding 2-functionalised-4,4'-biphenyldialdehydes in good yields (47-85%). The
Simona Rapposelli et al.
Archiv der Pharmazie, 344(6), 372-385 (2011-02-15)
Aldose reductase (ARL2) is the first enzyme in the polyol pathway which catalyzes the NADPH-dependent reduction of glucose to sorbitol. Its involvement on diabetic complications makes this enzyme a challenge therapeutic target widely investigated to limit and/or prevent them. On
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service