All Photos(1)
About This Item
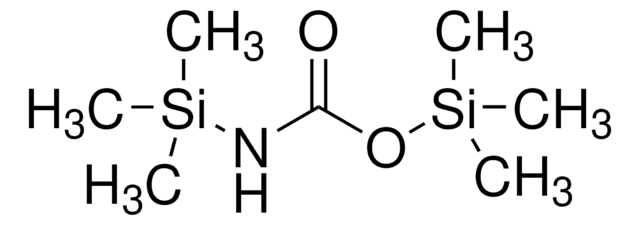
Empirical Formula (Hill Notation):
C7H15NOSi
CAS Number:
Molecular Weight:
157.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.46 (lit.)
bp
90 °C/20 mmHg (lit.)
density
0.983 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)N1CCCC1=O
InChI
1S/C7H15NOSi/c1-10(2,3)8-6-4-5-7(8)9/h4-6H2,1-3H3
InChI key
LUBVCBITQHEVCJ-UHFFFAOYSA-N
General description
1-(Trimethylsilyl)-2-pyrrolidinone has been prepared by refluxing a mixture of 2-pyrrolidinone, triethylamine and trimethylchlorosilane in benzene. It undergoes sulfenylation with phenyl disulfide to form the corresponding bissulfide as the major product.
Application
1-(Trimethylsilyl)-2-pyrrolidinone may be used for the preparation of 1-(diphenylphosphonio)pyrrolidin-2-one and 1-(1-adamantylcarbonyl)-2-pyrrolidinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
127.4 °F - closed cup
Flash Point(C)
53 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, conformational characteristics and anti-influenza virus A activity of some 2-adamantylsubstituted azacycles.
Setaki D, et al.
Bioorganic Chemistry, 34(5), 248-273 (2006)
Synthesis and antiviral activity evaluation of some new aminoadamantane derivatives. 2.
Kolocouris N, et al.
Journal of Medicinal Chemistry, 39(17), 3307-3318 (1996)
Efficient method for the preparation of carboxylic acid alkyl esters or alkyl phenyl ethers by a new-type of oxidation-reduction condensation using 2, 6-dimethyl-1, 4-benzoquinone and alkoxydiphenylphosphines.
Shintou T, et al.
Bulletin of the Chemical Society of Japan, 76(8), 1645-1667 (2003)
Sulfenylation and sulfinylation of lactams and imino ethers.
Zoretic PA, et al.
The Journal of Organic Chemistry, 43(7), 1379-1382 (1978)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service