369144
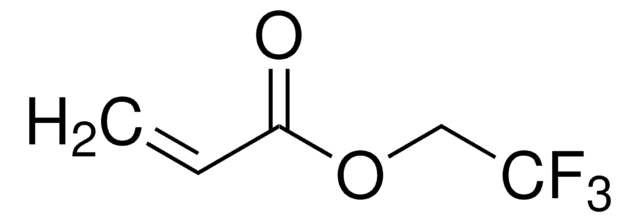
2-(Trifluoromethyl)acrylic acid
98%
Synonym(s):
TFMAA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2C=C(CF3)CO2H
CAS Number:
Molecular Weight:
140.06
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
mp
51-52 °C (lit.)
SMILES string
OC(=O)C(=C)C(F)(F)F
InChI
1S/C4H3F3O2/c1-2(3(8)9)4(5,6)7/h1H2,(H,8,9)
InChI key
VLSRKCIBHNJFHA-UHFFFAOYSA-N
Application
2-(Trifluoromethyl)acrylic acid (TFMAA) is a strong acid functional monomer, which shows a good performance in the solid-phase extraction of domoic acid. It can also be used in the preparation of molecularly imprinted polymers that show diastereoselectivity for cinchona alkaloids.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhengqiang Dai et al.
Journal of molecular modeling, 21(11), 290-290 (2015-10-27)
Recently, a series of computational and combinatorial approaches were employed to improve the efficiency of screening for optimal molecularly imprinted polymer (MIP) systems. In the present work, we investigated MIP systems based on enrofloxacin (ENRO) as the template molecule and
Molecularly imprinted fluorescent-shift receptors prepared with 2-(trifluoromethyl) acrylic acid
Matsui J, et al.
Analytical Chemistry, 72(14), 3286-3290 (2000)
Extraction of domoic acid from seawater and urine using a resin based on 2-(trifluoromethyl) acrylic acid
Piletska EV, et al.
Analytica Chimica Acta, 610(1), 35-43 (2008)
Ling Zhang et al.
Nature, 557(7703), 86-91 (2018-05-04)
The formation of condensed matter typically involves a trade-off between structural order and flexibility. As the extent and directionality of interactions between atomic or molecular components increase, materials generally become more ordered but less compliant, and vice versa. Nevertheless, high
Porkodi Kadhirvel et al.
Analytical and bioanalytical chemistry, 411(8), 1525-1536 (2019-02-03)
A molecularly imprinted polymer (MIP) was designed in order to allow the selective solid-phase extraction of carbamazepine (CBZ), an anticonvulsant and mood-stabilizing drug, at ultra-trace level from aqueous environmental samples. A structural analog of CBZ was selected as a dummy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service