176974
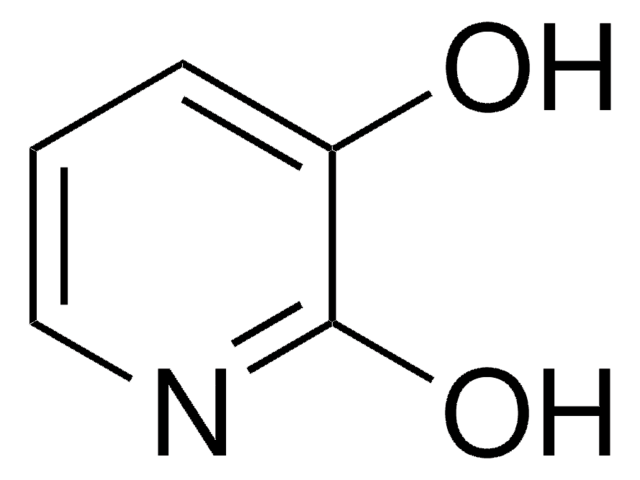
2,4-Dihydroxypyridine
97%
Synonym(s):
2,4-Pyridinediol, 3-Deazauracil, 4-Hydroxy-2-pyridone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO2
CAS Number:
Molecular Weight:
111.10
Beilstein:
108533
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
form
solid
mp
272-276 °C (lit.)
SMILES string
Oc1ccnc(O)c1
InChI
1S/C5H5NO2/c7-4-1-2-6-5(8)3-4/h1-3H,(H2,6,7,8)
InChI key
ZEZJPIDPVXJEME-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dihydroxypyridine (3-deazauracil) is a potent inhibitor of dihydrouracil dehydrogenase.
Application
2,4-Dihydroxypyridine (3-deazauracil) was used in the synthesis of diazaphenoxathiin skeleton.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Molgó et al.
Journal de pharmacologie, 16 Suppl 2, 109-144 (1985-01-01)
In this review the effects of aminopyridines and chemically related compounds are documented in an attempt to analyse the mechanism underlying their presynaptic actions at the vertebrate neuromuscular junction. Aminopyridines and related compounds are of particular interest because they greatly
K T Lin et al.
Therapeutic drug monitoring, 5(4), 491-496 (1983-01-01)
A rapid and simple procedure for liquid chromatographic analysis of plasma 3-deazauridine (3-DU), an antineoplastic agent, was developed. The plasma was extracted with methanolic silver acetate to remove interfering ultraviolet-absorbing materials and the 3-DU partially purified on a small anion
Maria Teresa Cocco et al.
European journal of medicinal chemistry, 38(1), 37-47 (2003-02-21)
Bis(pyridyl)methane derivatives 5-40 were obtained from the reaction of 4-hydroxy-2-pyridones 3 and 4 with aldehydes. Compounds 5-40 were evaluated for cytotoxic activity against a panel of 60 human cancer cell lines by the National Cancer Institute and some of them
F N Naguib et al.
Biochemical pharmacology, 38(9), 1471-1480 (1989-05-01)
One hundred and five nucleobase analogues were screened as inhibitors of dihydrouracil dehydrogenase (DHUDase, EC 1.3.1.2) from mouse liver. 5-Benzyloxybenzyluracil, 1-deazauracil (2,6-pyridinediol), 3-deazauracil (2,4-pyridinediol), 5-benzyluracil, 5-nitrobarbituric acid and 5,6-dioxyuracil (alloxan) were identified as potent inhibitors of this activity, with apparent
M T Cocco et al.
European journal of medicinal chemistry, 35(5), 545-552 (2000-07-12)
4-hydroxy-2-pyridone derivatives 2 were prepared by reaction of 3-amino-3-dialkylaminopropenoates with bis(2,4, 6-trichlorophenyl)malonate. These compounds were further reacted with a set of aldehydes to give bis(pyridyl)methanes 3 and 4. The newly synthesized compounds 2, 3 and 4 were evaluated in vitro
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service