All Photos(1)
About This Item
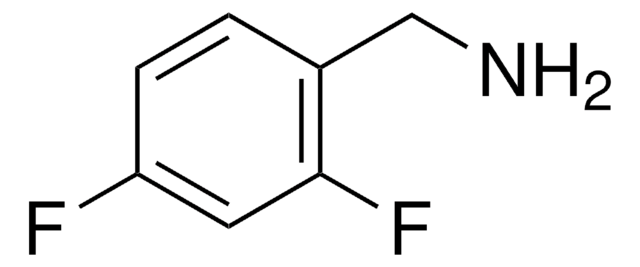
Linear Formula:
FC6H4CH2NH2
CAS Number:
Molecular Weight:
125.14
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39040407
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.517 (lit.)
bp
73-75 °C/13 mmHg (lit.)
density
1.095 g/mL at 25 °C (lit.)
SMILES string
NCc1ccccc1F
InChI
1S/C7H8FN/c8-7-4-2-1-3-6(7)5-9/h1-4H,5,9H2
InChI key
LRFWYBZWRQWZIM-UHFFFAOYSA-N
Application
2-Fluorobenzylamine was used in synthesis of 9-(2-fluorobenzyl)-6-(methylamino)-9H-purine, having anticonvulsant activity. It was also used in synthesis and study of structure-activity relationship of a series of substituted spirohydantoins.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Martin W Rowbottom et al.
Bioorganic & medicinal chemistry letters, 17(8), 2171-2178 (2007-03-14)
The design, synthesis, and SAR of a series of substituted spirohydantoins are described. Optimization of an in-house screening hit gave compounds that exhibited potent binding affinity and functional activity at MCH-R1.
9-(2-Fluorobenzyl)-6-(methylamino)-9H-purine hydrochloride. Synthesis and anticonvulsant activity.
J L Kelley et al.
Journal of medicinal chemistry, 29(7), 1133-1134 (1986-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service