A263
ATPA
solid
Synonym(s):
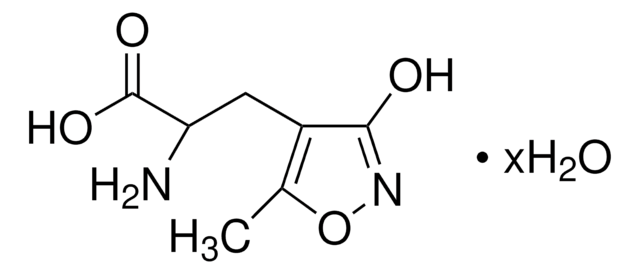
(RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16N2O4
CAS Number:
Molecular Weight:
228.25
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
form
solid
Quality Level
color
white
solubility
DMSO: 10 mg/mL
H2O: 2.5 mg/mL
SMILES string
CC(C)(C)c1onc(O)c1CC(N)C(O)=O
InChI
1S/C10H16N2O4/c1-10(2,3)7-5(8(13)12-16-7)4-6(11)9(14)15/h6H,4,11H2,1-3H3,(H,12,13)(H,14,15)
InChI key
PIXJURSCCVBKRF-UHFFFAOYSA-N
Gene Information
human ... GRIK1(2897)
rat ... Gria1(50592) , Grik1(29559) , Grin2a(24409)
Biochem/physiol Actions
Selective kainate receptor agonist.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S E Lauri et al.
Neuropharmacology, 41(8), 907-915 (2001-12-19)
The development of GluR5-selective kainate receptor ligands is helping to elucidate the functions of kainate receptors in the CNS. Here we have further characterised the actions of a GluR5 selective agonist, ATPA, and a GluR5 selective antagonist, LY382884, in the
Xiaoye Liu et al.
Frontiers in cell and developmental biology, 8, 590-590 (2020-08-01)
Migration of neutrophils across endothelial barriers to capture and eliminate bacteria is served as the first line of innate immunity. Bacterial virulence factors damage endothelium to produce inflammatory cytokines interacts with neutrophils. However, the mechanisms that behind endothelial-neutrophil interaction impact
Mai Marie Nielsen et al.
Molecular pharmacology, 63(1), 19-25 (2002-12-19)
Only a few agonists exhibit selectivity between the AMPA and the kainate subtypes of the glutamate receptor. The most commonly used kainate receptor preferring agonist, (S)-2-amino-3-(5-tert-butyl-3-hydroxy-4-isoxazolyl)propionic acid [(S)-ATPA], is an (R,S)-2-amino-3-(5-methyl-3-hydroxy-4-isoxazolyl)propionic acid (AMPA) derivative in which the methyl group at
V R J Clarke et al.
Neuropharmacology, 42(7), 889-902 (2002-06-19)
Kainate receptors are involved in a variety of synaptic functions in the CNS including the regulation of excitatory synaptic transmission. Previously we described the depressant action of the GLU(K5) selective agonist (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid (ATPA) on synaptic transmission in the Schaffer
Marie-Louise Lunn et al.
Journal of medicinal chemistry, 46(5), 872-875 (2003-02-21)
Two X-ray structures of the GluR2 ligand-binding core in complex with (S)-2-amino-3-(5-tert-butyl-3-hydroxy-4-isoxazolyl)propionic acid ((S)-ATPA) have been determined with and without Zn(2+) ions. (S)-ATPA induces a domain closure of ca. 21 degrees compared to the apo form. The tert-butyl moiety of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service