All Photos(1)
About This Item
Linear Formula:
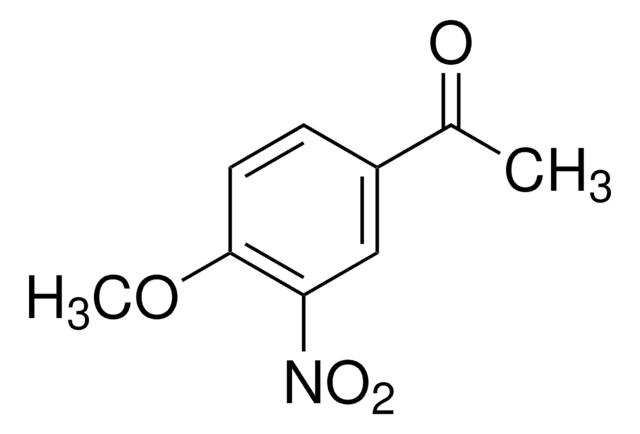
ClC6H3(NO2)COCH3
CAS Number:
Molecular Weight:
199.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
99-101 °C (lit.)
functional group
chloro
SMILES string
CC(=O)c1ccc(Cl)c(c1)[N+]([O-])=O
InChI
1S/C8H6ClNO3/c1-5(11)6-2-3-7(9)8(4-6)10(12)13/h2-4H,1H3
InChI key
YEVPHFIFGUWSMG-UHFFFAOYSA-N
General description
4′-Chloro-3′-nitroacetophenone is the intermediate formed during the synthesis of 4-chloro-3-nitrostyrene. It participates in deamination reaction of 4-chloro-5- and -3-nitro-2-aminoacetophanones.
Application
4′-Chloro-3′-nitroacetophenone was used to prepare starting reagent for the synthesis of 6- and 7-acetyl-3-methyl-2-quinoxalinecarboxamide1,4-dioxides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J P Dirlam et al.
Journal of medicinal chemistry, 21(5), 483-485 (1978-05-01)
The synthesis, separation, and structure determination of 6- and 7-acetyl--3-methyl-2-quinoxalinecarboxamide 1,4-dioxides are reported together with a comparison of their antibacterial activity. The structural assignment of these 6- and7-acetyl isomers was based on NMR analysis of related mono-N-oxide derivatives, which were
Cinnolines; the preparation of 4-chloro-2-amino-acetophenone and related 4-hydroxycinnolines.
C M ATKINSON et al.
Journal of the Chemical Society, 232-237 (1947-02-01)
Synthesis of 4-chloro-3-nitrostyrene.
Strantzalis N, et al.
Polym. Bull., 15(5), 431-438 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service