186708
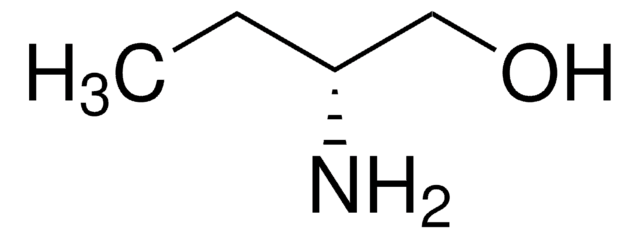
(S)-(+)-2-Amino-3-methyl-1-butanol
96%
Synonym(s):
L-Valinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH(NH2)CH2OH
CAS Number:
Molecular Weight:
103.16
Beilstein:
1719137
EC Number:
MDL number:
UNSPSC Code:
12352104
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
optical activity
[α]25/D +10°, c = 10 in H2O
optical purity
ee: 95% (GLC)
refractive index
n20/D 1.4548 (lit.)
bp
81 °C/8 mmHg (lit.)
mp
30-32 °C (lit.)
density
0.926 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
storage temp.
2-8°C
SMILES string
CC(C)[C@H](N)CO
InChI
1S/C5H13NO/c1-4(2)5(6)3-7/h4-5,7H,3,6H2,1-2H3/t5-/m1/s1
InChI key
NWYYWIJOWOLJNR-RXMQYKEDSA-N
Related Categories
Application
(S)-(+)-2-Amino-3-methyl-1-butanol can be used to prepare:
- Imines and oxazolines by reacting with aldehydes and nitriles, respectively.
- Chiral oxazoline derived multidentate ligands containing cyclophosphazene moiety.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tetrahedron Letters, 34, 2015-2015 (1993)
Journal of the Chemical Society. Perkin Transactions 1, 192-192 (1989)
Enantioselective palladium catalysed allylic substitution with thienyl oxazoline ligands
Frost CG and Williams JMJ
Tetrahedron Letters, 34(12), 2015-2018 (1993)
Asymmetric synthesis of alpha-substituted o-methoxybenzyl alcohols via stereoselective additions to kinetically resolved o-anisaldehyde (tricarbonyl) chromium
Davies SG and Goodfellow CL
Journal of the Chemical Society. Perkin Transactions 1, 1, 192-194 (1989)
Dheeraj Kumar et al.
Dalton transactions (Cambridge, England : 2003), 43(37), 13899-13912 (2014-08-12)
Chiral oxazoline based bi and hexadentate ligands built on cyclophosphazene cores have been synthesized and characterized. (NPPh2)2[NP(m-OC6H4C(O)OCH3)2] (1) was prepared by the reaction of gem-(NPPh2)2(NPCl2) with methyl-3-hydroxy benzoate in the presence of Cs2CO3. Compound 1 was converted to the dicarboxylic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service