160660
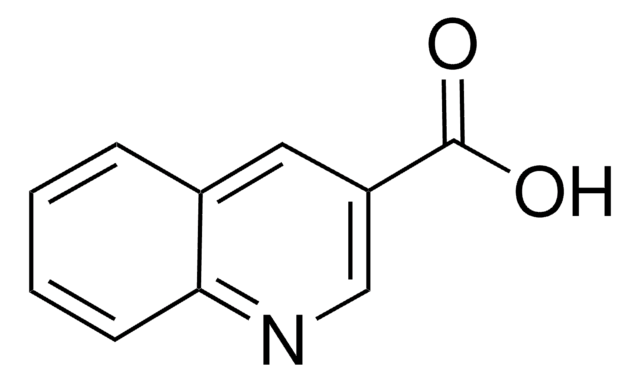
Quinaldic acid
98%
Synonym(s):
2-Quinolinecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
126322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
156-158 °C (lit.)
SMILES string
OC(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)
InChI key
LOAUVZALPPNFOQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N D Priestley et al.
Bioorganic & medicinal chemistry, 4(7), 1135-1147 (1996-07-01)
Specifically 13C-labeled quinoline-2-carboxylate derivatives were synthesized from quinoline and used to study the biosynthesis of thiostrepton in a strain of Streptomyces laurentii. 13C NMR analysis of thiostrepton recovered after feeding methyl (RS)-[11-13C]-4-(1-hydroxyethyl)quinoline-2-carboxylate or methyl [11-13C]-4-acetylquinoline-2-carboxylate showed conclusively that these compounds
Pavel Bobal et al.
Molecules (Basel, Switzerland), 17(2), 1292-1306 (2012-02-02)
In this study a one step method for the preparation of substituted anilides of quinoline-2-carboxylic acid was developed. This efficient innovative approach is based on the direct reaction of an acid or ester with substituted anilines using microwave irradiation. The
K Jhamandas et al.
Brain research, 529(1-2), 185-191 (1990-10-08)
Certain products of tryptophan metabolism interact with excitatory amino acid receptors to produce or protect against excitotoxicity. In this study, the action of several tryptophan metabolites, yielded by the kynurenine pathway, on cortical cholinergic toxicity was evaluated following focal injection
Mode of action of hypoglycemic agents. 3. Studies on 5-methoxy indole-2-carboxylic acid and quinaldic acid.
J Reed et al.
The Journal of biological chemistry, 245(20), 5297-5303 (1970-10-25)
Zhao-Qing Du et al.
Journal of Cancer, 11(15), 4614-4624 (2020-06-04)
Platelet-derived growth receptor α (PDGFRα) is a key factor in many pathophysiological processes. The expression level of PDGFRα is significantly elevated in the early stage of liver development and maintained at a lower level in adult normal livers. In this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service