SAB2108553
Anti-FAH
IgG fraction of antiserum
About This Item
Productos recomendados
origen biológico
rabbit
Nivel de calidad
conjugado
unconjugated
forma del anticuerpo
IgG fraction of antiserum
tipo de anticuerpo
primary antibodies
clon
polyclonal
Formulario
buffered aqueous solution
mol peso
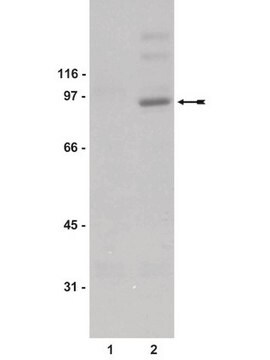
46 kDa
reactividad de especies
dog, horse, human, mouse, rat
concentración
0.5-1 mg/mL
técnicas
immunoblotting: suitable
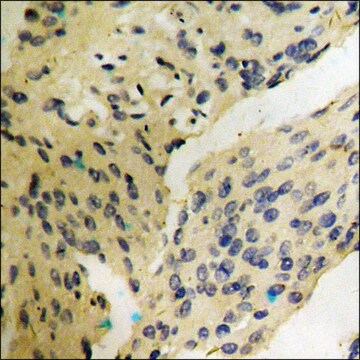
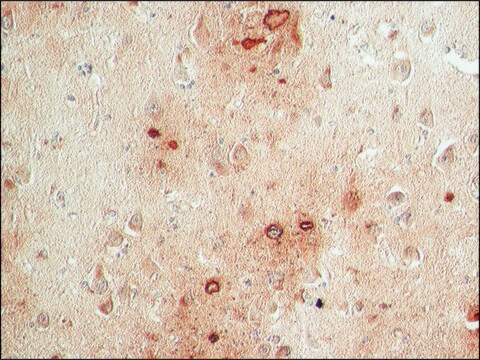
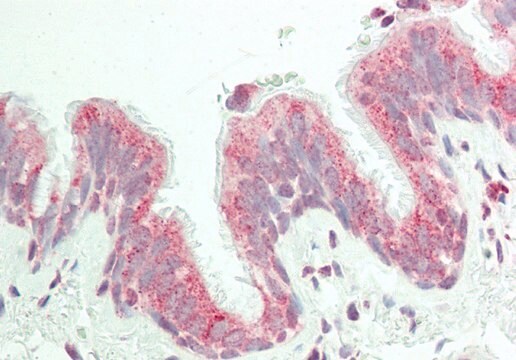
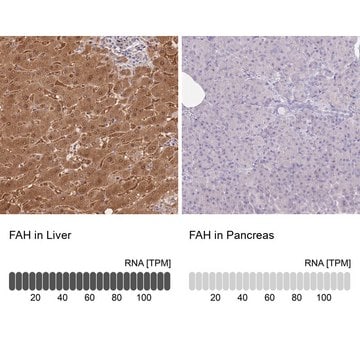
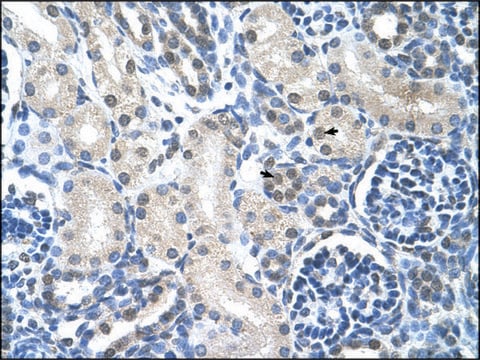
immunohistochemistry: suitable
nº de acceso
NM_000137
Nº de acceso UniProt
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
modificación del objetivo postraduccional
unmodified
Información sobre el gen
human ... FAH(2184)
Descripción general
Inmunógeno
Acciones bioquímicas o fisiológicas
Secuencia
Forma física
Cláusula de descargo de responsabilidad
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico