C9879
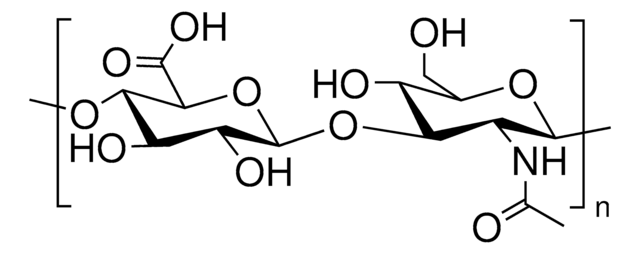
Bovine Collagen Type I
from bovine achilles tendon, powder, suitable for substrate for collagenase
Sinónimos:
Collagen powder
About This Item
Productos recomendados
product name
Collagen from bovine achilles tendon, powder, suitable for substrate for collagenase
origen biológico
bovine Achilles tendon
formulario
powder
técnicas
ELISA: suitable
activity assay: suitable
idoneidad
suitable for substrate for collagenase
Nº de acceso UniProt
temp. de almacenamiento
2-8°C
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- the detection of collagenase activity
- as a reference sample in the thermal analysis study of human bone using differential scanning calorimetry, thermogravimetry, gas chromatography and Fourier transform infrared spectroscopy
- as a substrate for developing a simple assay for determining collagen degradation in vitro
- a study to examine the binding activity of the integral glycoprotein dipeptidyl peptidase IV to insoluble type I collagen by solid-phase enzyme-linked immunosorbent assay
Acciones bioquímicas o fisiológicas
Nota de preparación
Reconstitución
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico