Y0001554
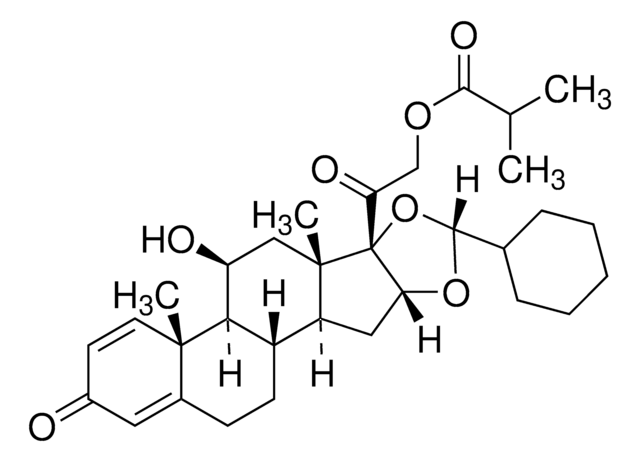
Ciclesonide containing impurity A
European Pharmacopoeia (EP) Reference Standard
About This Item
Productos recomendados
origen biológico
synthetic
grado
pharmaceutical primary standard
Agency
EP
familia API
ciclesonide
envase
pkg of 15 mg
fabricante / nombre comercial
EDQM
condiciones de almacenamiento
protect from light
color
white
bp
665.0 °C/1.333 hPa (1229.0°F)
mp
209-211 °C (408—412°F)
solubilidad
water: <0.1 g/L
acetone: soluble
ethanol: soluble
densidad
1.23 g/cm3 at 20 °C (1.333 hPa)
aplicaciones
pharmaceutical (small molecule)
formato
neat
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
Descripción general
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Aplicación
Envase
Otras notas
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Choose from one of the most recent versions:
Certificados de análisis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico