D-915
Desalkylflurazepam solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 1 mL
fabricante / nombre comercial
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland)
concentración
1.0 mg/mL in methanol
técnicas
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicaciones
clinical testing
formato
single component solution
temp. de almacenamiento
−20°C
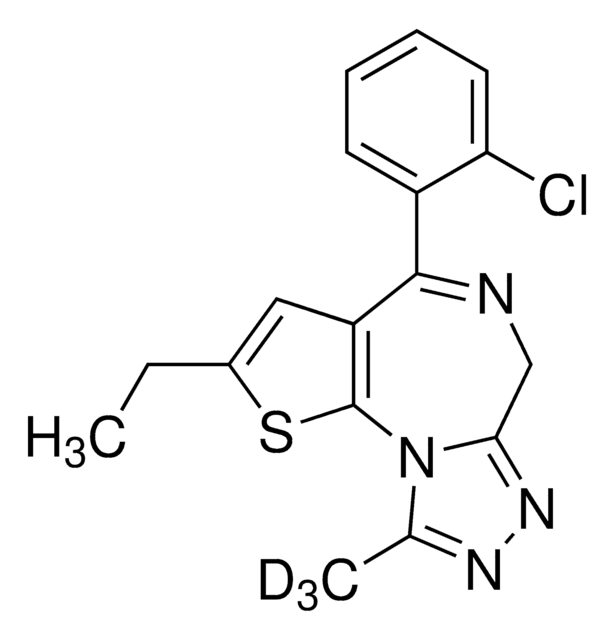
cadena SMILES
Fc1ccccc1C2=NCC(=O)Nc3ccc(Cl)cc23
InChI
1S/C15H10ClFN2O/c16-9-5-6-13-11(7-9)15(18-8-14(20)19-13)10-3-1-2-4-12(10)17/h1-7H,8H2,(H,19,20)
Clave InChI
UVCOILFBWYKHHB-UHFFFAOYSA-N
Descripción general
Aplicación
- Pharmacokinetic profiling: Research on flurazepam metabolites, including Desalkylflurazepam, utilizes high-performance liquid chromatography for detailed pharmacokinetic studies in rats, providing essential data for understanding drug behavior and metabolism (Lau et al., 1987).
- Neuroscience tool: Desalkylflurazepam is applied in neuroscience research to understand the dynamics of benzodiazepine binding on living cells, utilizing small ligands in fluorescence correlation spectroscopy, a method pivotal for real-time molecular interactions study (Hegener et al., 2002).
- Drug testing applications: The compound is used in the development of analytical techniques like ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS/MS) for the detection of benzodiazepines in hair, useful in workplace drug testing and forensic science (Ramírez Fernández et al., 2015).
- Toxicological analysis: Desalkylflurazepam serves as a reference standard in forensic toxicology to facilitate the rapid determination of benzodiazepines and their metabolites in biological samples, crucial for accurate and swift diagnostic purposes (Jeong et al., 2015).
- Immunosorbent assay applications: This benzodiazepine metabolite is instrumental in examining cross-reactivity of psychoactive substances in enzyme-linked immunosorbent assay (ELISA) techniques, enhancing drug testing capabilities in clinical settings (Cieri et al., 2024).
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Órganos de actuación
Eyes,Central nervous system
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
49.5 °F - closed cup
Punto de inflamabilidad (°C)
9.7 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico