ALD00564
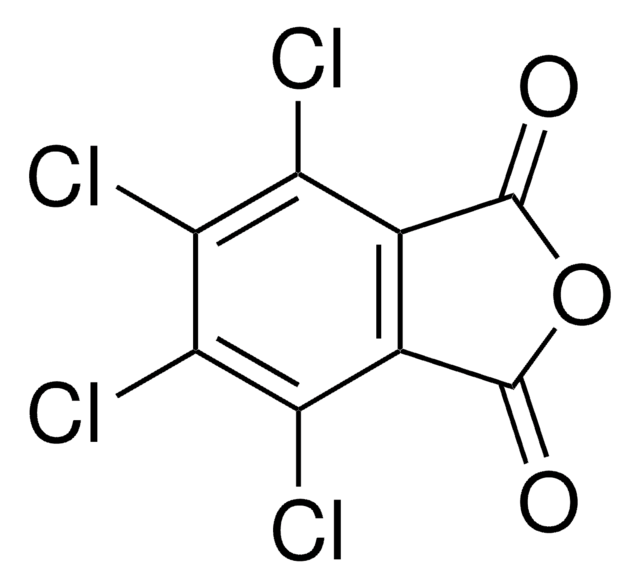
N-Hydroxytetrachlorophthalimide
Sinónimos:
4,5,6,7-Tetrachloro-2-hydroxy-1H-isoindole-1,3(2H)-dione, Tetrachloro-N-hydroxyphthalimide
About This Item
Productos recomendados
formulario
powder
Nivel de calidad
idoneidad de la reacción
reagent type: oxidant
cadena SMILES
O=C1N(O)C(C2=C(Cl)C(Cl)=C(Cl)C(Cl)=C21)=O
InChI
1S/C8HCl4NO3/c9-3-1-2(4(10)6(12)5(3)11)8(15)13(16)7(1)14/h16H
Clave InChI
UTRBHXSKVVPTLY-UHFFFAOYSA-N
Descripción general
Aplicación
Otras notas
Scalable and sustainable electrochemical allylic C–H oxidation
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Phil Baran develops ALD00564 reagent as a safe, cost-effective alternative for allylic oxidations and cross-coupling reactions.
Contenido relacionado
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico