718742
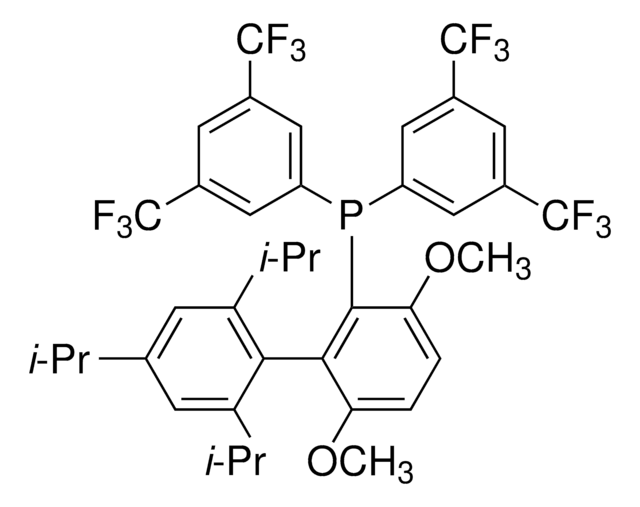
BrettPhos
98%
Sinónimos:
2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl
About This Item
Productos recomendados
Nivel de calidad
Análisis
98%
formulario
solid
idoneidad de la reacción
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Fluorinations
puntuación de productos alternativos más sostenibles
old score: 8
new score: 1
Find out more about DOZN™ Scoring
características de los productos alternativos más sostenibles
Waste Prevention
Atom Economy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-195 °C
grupo funcional
phosphine
categoría alternativa más sostenible
cadena SMILES
COc1c(P(C2CCCCC2)C3CCCCC3)c(c4c(C(C)C)cc(C(C)C)cc4C(C)C)c(OC)cc1
InChI
1S/C35H53O2P/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28/h19-25,27-28H,9-18H2,1-8H3
Clave InChI
WDVGNXKCFBOKDF-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
Aplicación
It can be used in:
- palladium-catalyzed trifluoromethylation of aryl chlorides
- Buchwald-Hartwig amination
- synthesis of 4-aryl and alkyl substituted, N6-alkylated pyridazine-3,6-diamines via a Buchwald protocol
Características y beneficios
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
nwg
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Contenido relacionado
Una herramienta para reacciones orgánicas proporciona relaciones estructura-actividad para reacciones químicas exclusivas destinadas al diseño y el control óptimos de la síntesis de moléculas pequeñas.
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico











![[2-(Dicyclohexylphosphino)-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl]gold(I) bis(trifluoromethanesulfonyl)imide](/deepweb/assets/sigmaaldrich/product/structures/361/949/e30e9505-889a-4ffd-9c57-f66a0a20b299/640/e30e9505-889a-4ffd-9c57-f66a0a20b299.png)


