391743
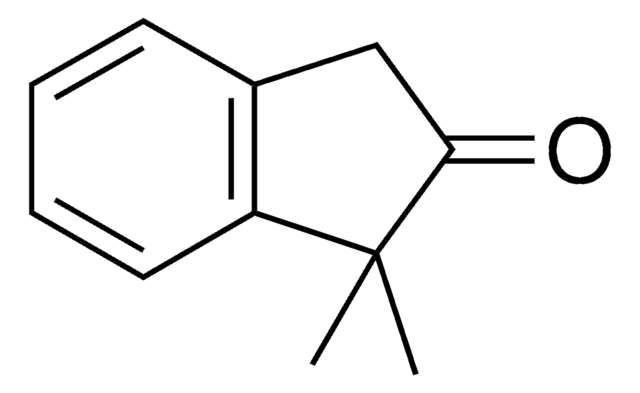
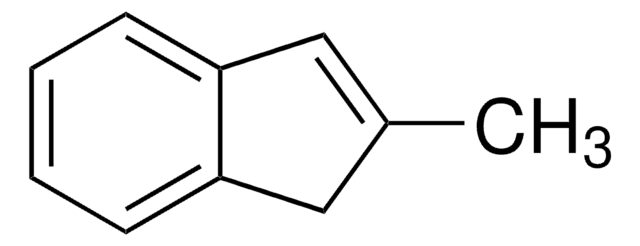
2-Methyl-1-indanone
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H10O
Número de CAS:
Peso molecular:
146.19
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Análisis
99%
formulario
liquid
índice de refracción
n20/D 1.555 (lit.)
bp
93-95 °C/4 mmHg (lit.)
densidad
1.064 g/mL at 25 °C (lit.)
cadena SMILES
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
Clave InChI
BEKNOGMQVKBMQN-UHFFFAOYSA-N
Descripción general
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
Aplicación
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Michał Rachwalski et al.
Chemical Society reviews, 42(24), 9268-9282 (2013-09-26)
Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which can be achieved either by invoking
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
Shaozhong Ge et al.
Journal of the American Chemical Society, 133(41), 16330-16333 (2011-09-16)
We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and charge-transfer interaction.
Luhowy R and Keehn PM.
Journal of the American Chemical Society, 99(11), 3797-3805 (1977)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico