292710
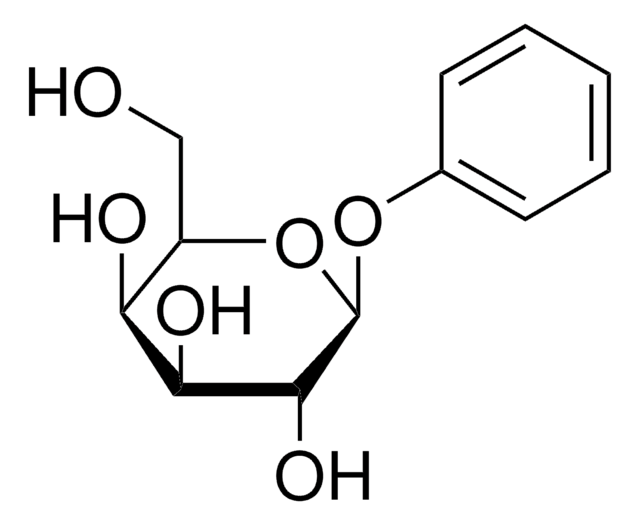
Phenyl β-D-glucopyranoside
≥95.0%
Sinónimos:
Phenyl beta-D-glucoside
About This Item
Productos recomendados
Análisis
≥95.0%
formulario
powder
actividad óptica
[α]25/D −70°, c = 1 in H2O
mp
176-178 °C (lit.)
cadena SMILES
OC[C@H]1O[C@@H](Oc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C12H16O6/c13-6-8-9(14)10(15)11(16)12(18-8)17-7-4-2-1-3-5-7/h1-5,8-16H,6H2/t8-,9-,10+,11-,12-/m1/s1
Clave InChI
NEZJDVYDSZTRFS-RMPHRYRLSA-N
Aplicación
- As a starting material for the synthesis of various derivatives of β-D-glucopyranosides with potential application as anti-HIV agents.
- As a model for glycosides in the gas phase for their spectroscopic investigation.
- As an internal standard in GC and GC-MS quantitative analyses.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico