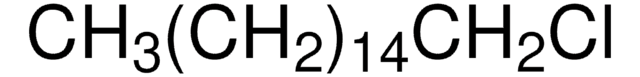24750
1-Chlorohexadecane
purum, ≥97.0% (GC)
Synonym(s):
Cetyl chloride, Hexadecyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)15Cl
CAS Number:
Molecular Weight:
260.89
Beilstein:
1748498
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
vapor pressure
129 mmHg ( 21 °C)
grade
purum
Assay
≥97.0% (GC)
refractive index
n20/D 1.449 (lit.)
bp
149 °C/1 mmHg (lit.)
mp
8-14 °C (lit.)
density
0.865 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCl
InChI
1S/C16H33Cl/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h2-16H2,1H3
InChI key
CLWAXFZCVYJLLM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
276.8 °F - closed cup
Flash Point(C)
136 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G L Murphy et al.
Applied and environmental microbiology, 53(1), 10-13 (1987-01-01)
The composition of phospholipids from Mycobacterium convolutum R22 was determined after growth at two temperatures (20 and 30 degrees C) with 1-chlorohexadecane as the substrate. Comparisons were made with the phospholipids of cells grown on n-hexadecane. Phosphatidylinositolmannosides and phosphatidylethanolamine (PE)
K Koike et al.
Applied and environmental microbiology, 65(12), 5636-5638 (1999-12-03)
A mutant Rhodococcus strain lacking the ability to utilize 1-chlorohexadecane was found to cis-desaturate aliphatic compounds, such as 1-chlorohexadecane, n-hexadecane, and heptadecanonitrile, yielding corresponding products with a double bond mainly at the ninth carbon from the terminal methyl groups. A
G L Murphy et al.
Journal of bacteriology, 156(3), 1158-1164 (1983-12-01)
The cellular fatty acid composition of Mycobacterium vaccae JOB5 and Mycobacterium convolutum R22 was examined after growth on n-alkanes and compared with the fatty acids of the organisms after growth on 1-chlorohexadecane and 1-chlorooctadecane. Growth on n-alkanes resulted in normal
H Curragh et al.
Microbiology (Reading, England), 140 ( Pt 6), 1433-1442 (1994-06-01)
The bacterium Rhodococcus rhodochrous NCIMB 13064, isolated from an industrial site, could use a wide range of 1-haloalkanes as sole carbon source but apparently utilized several different mechanisms simultaneously for assimilation of substrate. Catabolism of 1-chlorobutane occurred mainly by attack
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service