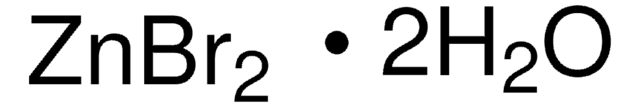8.18631
Zinc bromide
anhydrous for synthesis
Synonym(s):
Zinc bromide, Zinc dibromide
About This Item
Recommended Products
Quality Level
form
crystals
pH
4 (20 °C in H2O, saturated solution)
bp
697 °C/1013 mbar
mp
394 °C
solubility
4470 g/L
density
4.2 g/cm3 at 20 °C
storage temp.
2-30°C
InChI
1S/2BrH.Zn/h2*1H;/q;;+2/p-2
InChI key
VNDYJBBGRKZCSX-UHFFFAOYSA-L
Application
- A catalyst to synthesize β-hydroxy ketones by stereoselective semipinacol rearrangement of α-hydroxy epoxides.
- A safe brominating source for the oxidative bromination of alkenes and alkynes in combination with lead tetraacetate.
It can also be used to prepare silica-supported zinc bromide (ZnBr2/SiO2) composite, which is used as a catalyst to synthesize:
- Ynone derivatives via cross-coupling reaction of acid chlorides with terminal alkynes.
- 4,5-disubstituted-1,2,3-(NH)-triazole derivatives by one-pot cross-coupling and 1,3-dipolar cycloaddition reaction of acid chlorides, terminal alkynes, and sodium azide.
Analysis Note
Water (K. F.): ≤ 2.0 %
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service