All Photos(1)
About This Item
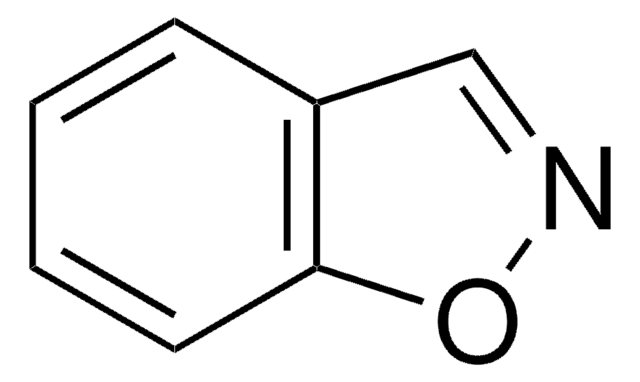
Empirical Formula (Hill Notation):
C8H6O
CAS Number:
Molecular Weight:
118.13
Beilstein:
107704
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.566 (lit.)
bp
173-175 °C (lit.)
density
1.072 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
c1ccc2occc2c1
InChI
1S/C8H6O/c1-2-4-8-7(3-1)5-6-9-8/h1-6H
InChI key
IANQTJSKSUMEQM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Timothy Lynagh et al.
The Journal of biological chemistry, 286(51), 43913-43924 (2011-10-29)
Ivermectin is an anthelmintic drug that works by activating glutamate-gated chloride channel receptors (GluClRs) in nematode parasites. GluClRs belong to the Cys-loop receptor family that also includes glycine receptor (GlyR) chloride channels. GluClRs and A288G mutant GlyRs are both activated
Preetpal Singh Sidhu et al.
Journal of medicinal chemistry, 56(12), 5059-5070 (2013-05-31)
We recently designed a group of novel exosite-2-directed sulfated, small, allosteric inhibitors of thrombin. To develop more potent inhibitors, monosulfated benzofuran tri- and tetrameric homologues of the parent designed dimers were synthesized in seven to eight steps and found to
Xiaomin Liu et al.
Chemical communications (Cambridge, England), 47(43), 11957-11959 (2011-10-05)
The concentration quenching threshold of upconversion luminescence was broken through for the first time via a designed strategy: spatial separation of the emitter doping area.
Javarappa Rangaswamy et al.
Bioorganic & medicinal chemistry letters, 22(14), 4773-4777 (2012-06-15)
In search for a new antioxidant and antimicrobial agent with improved potency, we synthesized a series of benzofuran based 1,3,5-substituted pyrazole analogues (5a-l) in five step reaction. Initially, o-alkyl derivative of salicyaldehyde readily furnish corresponding 2-acetyl benzofuran 2 in good
Highly diastereoselective multicomponent cascade reactions: efficient synthesis of functionalized 1-indanols.
Jun Jiang et al.
Angewandte Chemie (International ed. in English), 52(5), 1539-1542 (2013-01-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service