904805
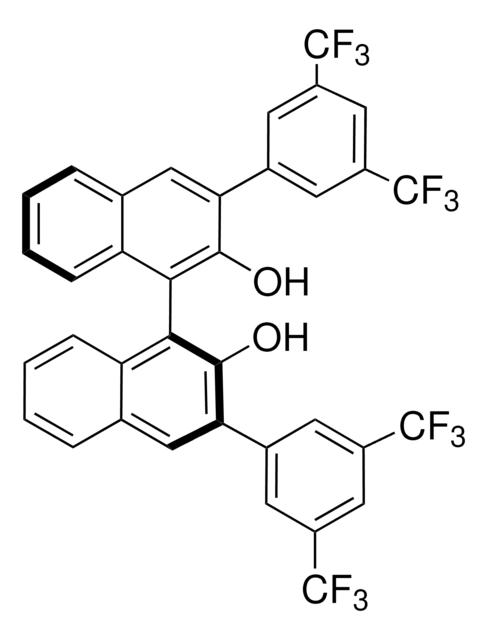
((S)-3-(3,5-Bis(trifluoromethyl)phenyl)-1-(2′-(3-(3,5-bis(trifluoromethyl)phenyl)ureido)-[1,1′-binaphthalen]-2-yl)-1-isopropylurea
≥95%
Synonym(s):
Gouverneur fluorination catalyst
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C41H28F12N4O2
CAS Number:
Molecular Weight:
836.67
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder or crystals
mp
144-160 °C
Related Categories
Application
((S)-3-(3,5-Bis(trifluoromethyl)phenyl)-1-(2′-(3-(3,5-bis(trifluoromethyl)phenyl)ureido)-[1,1′-binaphthalen]-2-yl)-1-isopropylurea is a hydrogen bonding phase-transfer catalyst capable of activating CsF for the asymmetric nucleophilic fluorination of sulfoniums.1
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gabriele Pupo et al.
Science (New York, N.Y.), 360(6389), 638-642 (2018-05-12)
Common anionic nucleophiles such as those derived from inorganic salts have not been used for enantioselective catalysis because of their insolubility. Here, we report that merging hydrogen bonding and phase-transfer catalysis provides an effective mode of activation for nucleophiles that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(R)-N-[(1R,2R)-2-(3-(3,5-Bis(trifluoromethyl)phenyl)ureido)cyclohexyl]-tert-butyl-sulfinamide 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)
![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)
![(S)-2-[[3,5-Bis(trifluoromethyl)phenyl]thioureido]-N-benzyl-N,3,3-trimethylbutanamide 97%](/deepweb/assets/sigmaaldrich/product/structures/373/888/118b46f2-6c2e-4a87-8266-c4dbcd5db51f/640/118b46f2-6c2e-4a87-8266-c4dbcd5db51f.png)