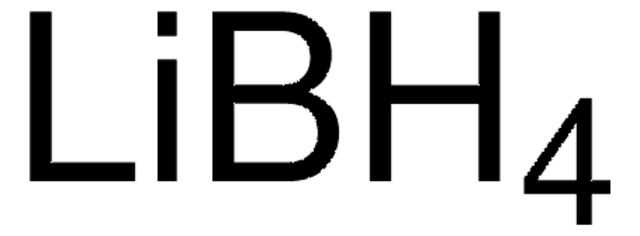593702
Lithium aluminum hydride solution
2.0 M in THF
Synonym(s):
LAH, Lithium alanate, Lithium tetrahydroaluminate
About This Item
Recommended Products
Quality Level
reaction suitability
reagent type: reductant
concentration
2.0 M in THF
density
0.906 g/mL at 25 °C
storage temp.
2-30°C
SMILES string
[Li+].[AlH4-]
InChI
1S/Al.Li.4H/q-1;+1;;;;
InChI key
OCZDCIYGECBNKL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- The preparation of thermoplastic polyester polyamides from oleic acid
- Lithium-polymer batteries
- Hydrodefluorination of gem-difluoromethylene derivatives
- Asymmetric aldol reactions
- Synthesis of Li-Al-N-H composites with hydrogen absorption / desorption properties
LAH is a powerful reducing agent for many different reduction reactions such as that of ketones to alcohols
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3 - Water-react 1
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
-13.0 °F - closed cup
Flash Point(C)
-25 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service